
- Change Your Password

Search this Resource
Table of contents.
- Alternative/Holistic Medicine
- Anesthesia & Pain Management
- Animal Welfare
- Award Lectures
- Canine Myocardial Disease
- Cardiac Disease & Examination
- Cardiac Disease Diagnosis
- Arrhythmia Diagnosis & Management
- Pericardial Disease
- Interventional Cardiac Catheterization
- Feline Myocardial Diseases
- Valvular Heart Disease
- Clinical Immunology
- Clinical Pathology
- Critical Care
- Dermatology
- Diagnostic Imaging
- Emergency Medicine
- Endocrinology
- Exotics/Wildlife
- Fracture Surgery
- Gastrointestinal Surgery
- Joint Surgery
- NAVC How I Treat…
- Neurology/Surgery
- Oncologic Surgery
- One Health/Zoonotic
- Ophthalmology
- Pharmacology
- Physical Rehabilitation
- Reproduction
- Thoracic Surgery
- Urogenital Surgery
- Working Dog
Benefits and Limitations
The electrocardiogram (ECG or EKG) provides a graphic representation of the electrical depolarization and repolarization processes of the cardiac muscle, as "viewed" from the body surface. The amplitude of these electrical potential differences between various points on the body is measured in millivolts (mV) and their duration in seconds. The ECG can provide information on heart rate, rhythm, and intracardiac conduction; it may also reveal evidence of specific chamber enlargement, myocardial disease or ischemia, pericardial disease, certain electrolyte imbalances, and some drug toxicities. But note that although the ECG is a valuable part of the cardiac evaluation, it cannot determine if congestive heart failure is present, or (in itself) predict whether an animal will survive procedures requiring anesthesia, nor can it provide much information on the strength (or even presence) of cardiac contractions.
Sinus rhythm is the normal cardiac rhythm, described above. The P waves are positive in the caudal leads (II and aVF), the P-Q intervals are consistent and the R-R intervals occur regularly, with less than 10% variation in timing. Normally, the QRS complexes are narrow and upright in leads II and aVF; however, if an intraventricular conduction disturbance or ventricular enlargement pattern is present, they may be wide and abnormally shaped.
Sinus bradycardia is a rhythm that originates in the sinus node and is conducted normally but has too slow a rate, while sinus tachycardia also originates in the sinus node and is conducted normally but is too rapid.
Sinus arrhythmia is characterized by a cyclical slowing and speeding of the sinus rate, most commonly associated with respiration. The rate tends to increase on inspiration and decrease with expiration because of changes in vagal tone. Often, there is an accompanying change in P wave configuration (wandering pacemaker) with the P waves becoming taller and spiked during inspiration and flatter in expiration. Marked sinus arrhythmia occurs in some animals with chronic pulmonary disease. Sinus arrhythmia is a normal rhythm variation . It is commonly seen in dogs, but not often in the clinical setting in normal cats. However, cats frequently have sinus arrhythmia when relaxed or sleeping.

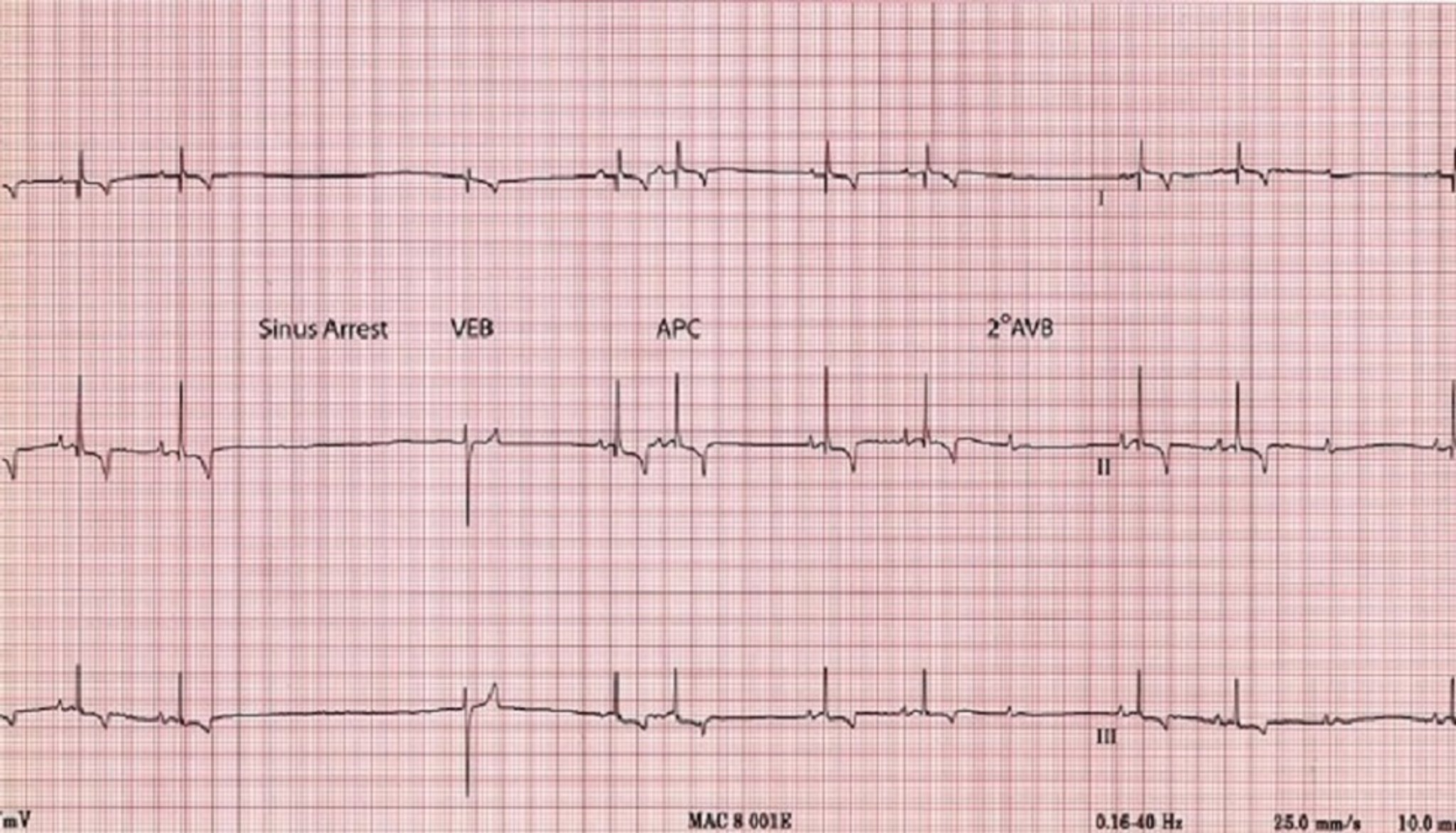
Sinus arrest is a cessation of sinus node activity lasting at least twice as long as the patient's longest expected R-R interval. The resulting pause in heart rate is interrupted by either an escape beat or resumption of sinus activity. Fainting or weakness may result during these pauses.
Conduction blocks in the major ventricular conduction system also disturb the normal activation process and result in altered QRS configurations. The portion of the ventricles served by the diseased bundle branch is activated late and slowly, resulting in widening of the QRS with the terminal forces oriented toward the area of delayed activation.
Rhythm Disturbances
Impulses originating from outside the sinus node are abnormal and create an arrhythmia (dysrhythmia). Abnormal or ectopic impulses are described based on their site of origin (atrial, junctional, supraventricular, ventricular). They are also characterized by timing , that is, whether they occur earlier than the next expected sinus impulse ( premature ) or whether they occur late ( escape ), as a rescue mechanism. Abnormal premature impulses (complexes) may occur singly or in multiples. Groups of three or more comprise an episode of tachycardia ; bouts of tachycardia may be brief (paroxysmal tachycardia) or quite prolonged (sustained tachycardia). A bigeminal pattern occurs when each normal QRS is followed by a premature complex; the origin of the premature complexes determines whether the rhythm is atrial or ventricular bigeminy.

Supraventricular (atrial, junctional) premature complexes originate above the AV node, in either the atrium or the AV junctional (near the AV node) area; however, since they are conducted through the ventricles in the normal manner, their QRS configuration is normal (unless an intraventricular conduction disturbance is also present). Atrial premature complexes are preceded by an abnormal P wave (either positive, negative or biphasic).
Ventricular premature complexes (VPCs or PVCs) originate below the AV node and do not activate the ventricles by the normal pathway; therefore, they have an abnormal ECG configuration. Ventricular ectopic complexes are also wider than the normal QRS complexes because of their slower conduction through ventricular muscle. When the configuration of VPCs or tachycardia in a patient is consistent, the complexes are described as being uniform or unifocal. When the VPCs occurring in an individual have differing configurations, they are said to be multiform. Increased electrical instability of the heart is thought to accompany multiform VPCs or tachycardia. Ventricular tachycardia defines a rapid series of VPCs (greater than 100 beats/minute in the dog, for example). The R-R interval is usually regular, although some variation is not uncommon. Sinus P waves may be seen superimposed on or between the ventricular complexes; they are unrelated to the VPCs because the AV node and/or ventricles are in the refractory period (physiologic AV dissociation).

Atrial fibrillation ("delirium cordis") is a common arrhythmia characterized by rapid, chaotic electrical activation of the atria. There are no P waves on the ECG; rather, the baseline usually shows irregular undulations (fibrillation waves). Since there is no organized electrical activity, meaningful atrial contraction is absent. The AV node, being constantly bombarded with these disorganized electrical impulses, conducts as many as possible to the ventricles. The (ventricular) heart rate is, therefore, determined by how many impulses the AV node can conduct. Atrial fibrillation results in an irregular heart rhythm, which is usually quite rapid. Most often, the QRS complexes appear normal in configuration, since the normal intraventricular conduction pathway is used. Atrial fibrillation tends to be a consequence of significant atrial disease and enlargement in small animals.

Atrio - ventricular (AV) conduction blocks may result from therapy with certain drugs, high vagal tone, and organic disease of the AV node and/or ventricular conduction system. AV blocks are also called "Heart Blocks."

Matthew W. Miller, DVM, MS, DACVIM (Cardiology) College of Veterinary Medicine and Biomedical Sciences Texas A&M University College Station, TX, USA
UNIVERSITY OF ILLINOIS URBANA-CHAMPAIGN
Header Main
College of Veterinary Medicine
Home » Pet Columns » Pacemakers Solve Canine Heart Problems
Pacemakers Solve Canine Heart Problems
![wandering pacemaker in dogs [patient with pacemaker is very active]](https://vetmed.illinois.edu/wp-content/uploads/2021/04/pc-pacemaker-lucy4.jpg)
‘They are more common than you think’
The heart is essential to the body, regardless of the species. Luckily, when dogs have heart problems, veterinary cardiologists, like Dr. Ryan Fries at the University of Illinois Veterinary Teaching Hospital in Urbana, are able to keep things ticking along.
Pacemakers have been used in human medicine since the early 1960s. In the late 1980s, Dr. David Sisson at the University of Illinois became one of the first veterinary cardiologists to place intravenous pacemakers in canine patients. Currently, the university’s Veterinary Teaching Hospital is the only veterinary facility in the state of Illinois that offers this procedure.
In dogs, pacemakers are used both as a life-saving intervention and to improve quality of life.
How Pacemakers Work
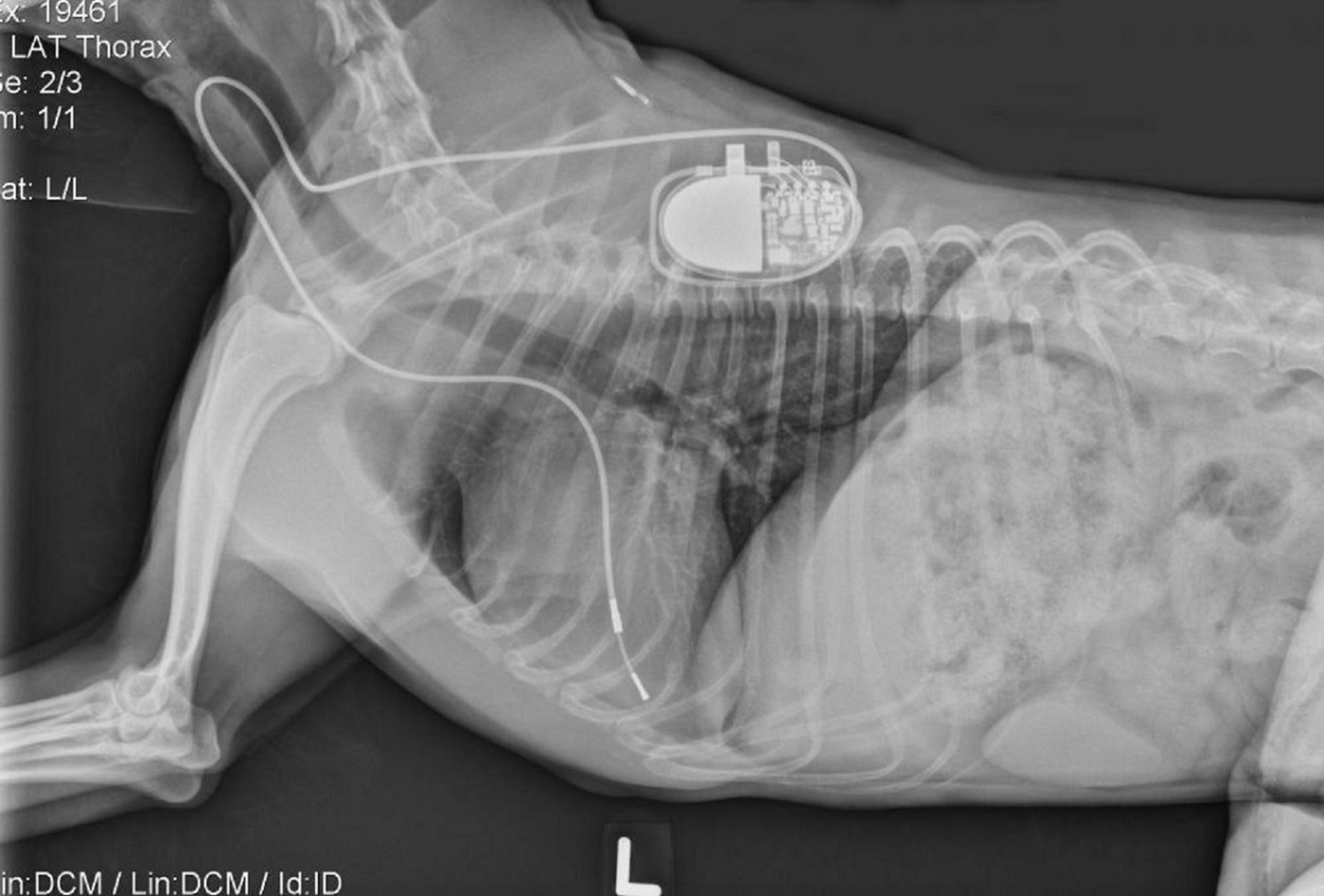
“A pacemaker is made up of two parts,” says Dr. Fries. “One part consists of a generator, a lithium battery, and a computer chip that we can program to meet the dog’s needs. The other part consists of wires, called leads, that extend from the generator through veins in the neck and are attached to the inside of the heart.”
The pacemaker is activated when the dog’s heart rate slows below the acceptable range set by the veterinarian, generally between 80 and 120 beats per minute. When the pacemaker kicks on, it stimulates contractions of the heart until the heart’s rhythm is reset and can continue on its own.
Cardiologists like Dr. Fries place pacemakers while the dog is under anesthesia. The surgery is most commonly done using minimally invasive techniques. The equipment used is the same that’s used in humans, but the procedure is much more affordable: “The entire procedure typically costs between $3,500 and $4,000, which is consistent with other specialized veterinary procedures,” says Dr. Fries.
How Pacemakers Are Placed
“A small incision is made in the dog’s neck, and the leads are fed through the external jugular vein, the same vein used to draw blood. Once the leads are in, the generator is tucked in the skin and stitched up,” explains Dr. Fries.
![wandering pacemaker in dogs [radiographs showing a pacemaker in place]](https://vetmed.illinois.edu/wp-content/uploads/2021/04/pc-fries-pacemaker-xrays.jpg)
“Dogs might benefit from a pacemaker if they have an arrhythmia (abnormal heart rhythm) or a heart rate that is too slow to support the dog in daily activities,” says Dr. Fries. “Some arrhythmias can stop the heart and be life-threatening. Other heart conditions may simply impede the dog’s ability to exercise and live a normal life.”
How Pacemakers Help Dogs
A classic presentation of a non-life-threatening heart problem occurs when an otherwise healthy dog suddenly faints while doing routine activities because of reduced blood flow from a slow or irregular heartbeat.

Though not associated with a specific breed, an advanced atrioventricular (AV) block—a condition in which the impulse that causes contractions in the heart’s atrium is not conveyed appropriately to the ventricle—can be treated with a pacemaker.
“Pacemakers can be a long-term solution and often allow the dog to return to full capacity. If placed early in a dog’s life, the battery may be used enough to wear out. However, the battery can be replaced quite easily,” says Dr. Fries.
Follow-Up Care
A dog with a pacemaker will likely need checkups every six months, alternating visits between a primary care veterinarian and a veterinary cardiologist, according to Dr. Fries. If needed, the settings on the pacemaker can easily be reprogrammed by a veterinarian, who will adjust the computer program by placing a magnet over the skin. No surgery is necessary.
Following a month of rest after the surgery, dogs with pacemakers should be ready to resume normal activities. The only thing owners need to do is switch from a collar to a harness to keep pressure off the dog’s neck where the generator is.
“Pacemakers may offer the only treatment option that allows a dog to return to a normal life. We even put them in working animals that return to their jobs,” says Dr. Fries. “They are more common than you would think. There are no outward signs to tell the difference between a dog with or without one!”
If you have questions about pacemakers for dogs, contact your local veterinarian or the cardiology service at the University of Illinois Veterinary Teaching Hospital.
By Hannah Beers
Photographs courtesy of Lucy’s owner
More From Our Blog:

Cicadas: Pet Treat or Pet Threat?

When Does a Wildlife Baby Need Human Help?

Failure of Passive Transfer in Foals
Heart Disease: Conduction Abnormalities in Dogs and Cats
- ECG Waveform Abnormalities |
- Sinus Rhythm and Sinus Node Abnormalities |
- Atrioventricular Conduction Disturbances |
- Common Tachyarrhythmias |
- Antiarrhythmics |
ECG Waveform Abnormalities in Dogs and Cats
Chamber enlargement can be indicated by waveform abnormalities in dogs and cats; however, these abnormalities are commonly absent when there is chamber enlargement and are sometimes present when the heart is normal. In lead II in dogs and cats, wide or notched P waves suggest left atrial enlargement, whereas tall P waves suggest right atrial enlargement. Tall R waves in leads that have the positive electrode on the left and/or caudal aspect of the body (leads I, II, aVF, CV6LL, and CV6LU) are evidence of left ventricular enlargement. Wide QRS complexes can occur in animals with either right or left ventricular enlargement; however, they can also be due to conduction disturbances . Electrocardiography is very insensitive at identifying mild to moderate changes in chamber size and unacceptably insensitive for detecting severe enlargement. Although false-positive findings are less frequent than false-negative findings, they do occur. Consequently, the level of accuracy is unacceptable, and ECGs should therefore not be used to infer chamber enlargement.
Sinus Rhythm and Sinus Node Abnormalities in Animals
The sinus node initiates depolarization of the rest of the heart in a healthy animal, sets the normal rate and rhythm, and is called the normal pacemaker of the heart. It functions as the pacemaker because it is automatic (depolarizes on its own) and does so at a rate faster than the other automatic sites in the heart (AV node and Purkinje fibers). Normal sinus rhythm is regular and originates at the sinus node, indicated on the ECG by a P wave that precedes each normal QRS complex. The rate at which the sinus node fires varies tremendously from species to species and situation to situation. A healthy dog can have a heart rate in the teens when asleep and ≥250 bpm during maximal exercise. A healthy but stressed cat can have a heart rate of 240 bpm at rest in an examination room.
Sinus bradycardia is a regular sinus rhythm that is slower than expected for a given species and for the situation the animal is in. Sinus bradycardia may be noted in animals overdosed with anesthetic drugs or agents that can result in increased vagal tone (primary or secondary) or decreased sympathetic tone (eg, xylazine, beta-blocker), as well as in hypothermic animals, hypothyroid animals, animals with sick sinus syndrome (see below), or animals with increased vagal tone secondary to systemic disease (such as respiratory, neurologic, ocular, GI, or urinary tract disease). Treatment for sinus bradycardia is typically not needed unless clinical signs associated with the bradycardia, such as exercise intolerance, weakness, or collapse, are noted. In dogs and cats, administration of atropine (up to 0.04 mg/kg, IV, IM, or SC) may be considered for treatment of bradycardia. The initiating cause should also be corrected.
Sinus tachycardia is the finding of a regular sinus rhythm at a rate faster than normal but generally appropriate for the situation the animal is in (eg, stress, exercise, heart failure). If the rate is inappropriately high (eg, 200 bpm in an otherwise healthy dog at rest at home), another form of tachycardia (eg, atrial or ventricular) should be considered. Causes include stress (resulting in high sympathetic drive), exercise, hyperthyroidism, fever, pain, hypovolemia, cardiac tamponade, heart failure, and administration of agents that can increase the rate of sinus node discharge (eg, catecholamines). Treatment is targeted at resolving the underlying cause. Administration of a beta-blocker (eg, atenolol) might be considered.
Sinus arrhythmia results from irregular discharge of the sinus node most commonly associated with the respiratory cycle. The site of impulse formation remains the sinus node; however, the frequency of the discharge varies. Sinus arrhythmia is a normal finding in dogs; it is abnormal in cats in the hospital setting, although it appears to be more common in cats in their home environment. Respiratory sinus arrhythmia is characterized by an increase in heart rate with inspiration and a decrease with expiration. In dogs, sinus arrhythmia can also occur without being in sync with respiration, and is instead associated with variation in the intensity of vagal tone. It is abolished by decreased vagal tone resulting from excitement, exercise, or administration of vagolytic drugs such as atropine. Sinus arrhythmia may be associated with a wandering pacemaker, which is characterized on the ECG by taller P waves during higher rates and smaller P waves during lower rates.
Sinoatrial (SA) block occurs when the impulse from the SA node fails to be conducted through the surrounding tissue to the atria and ventricles. Thus, no P waves or QRS complexes are noted on the ECG, and the P-P interval surrounding the break in sinus rhythm is an exact multiple of the normal P-P interval. Sinoatrial block is often difficult to diagnose in dogs because sinus arrhythmia is common, resulting in a variable normal P-P interval.
Courtesy of Dr. Mark D. Kittleson.
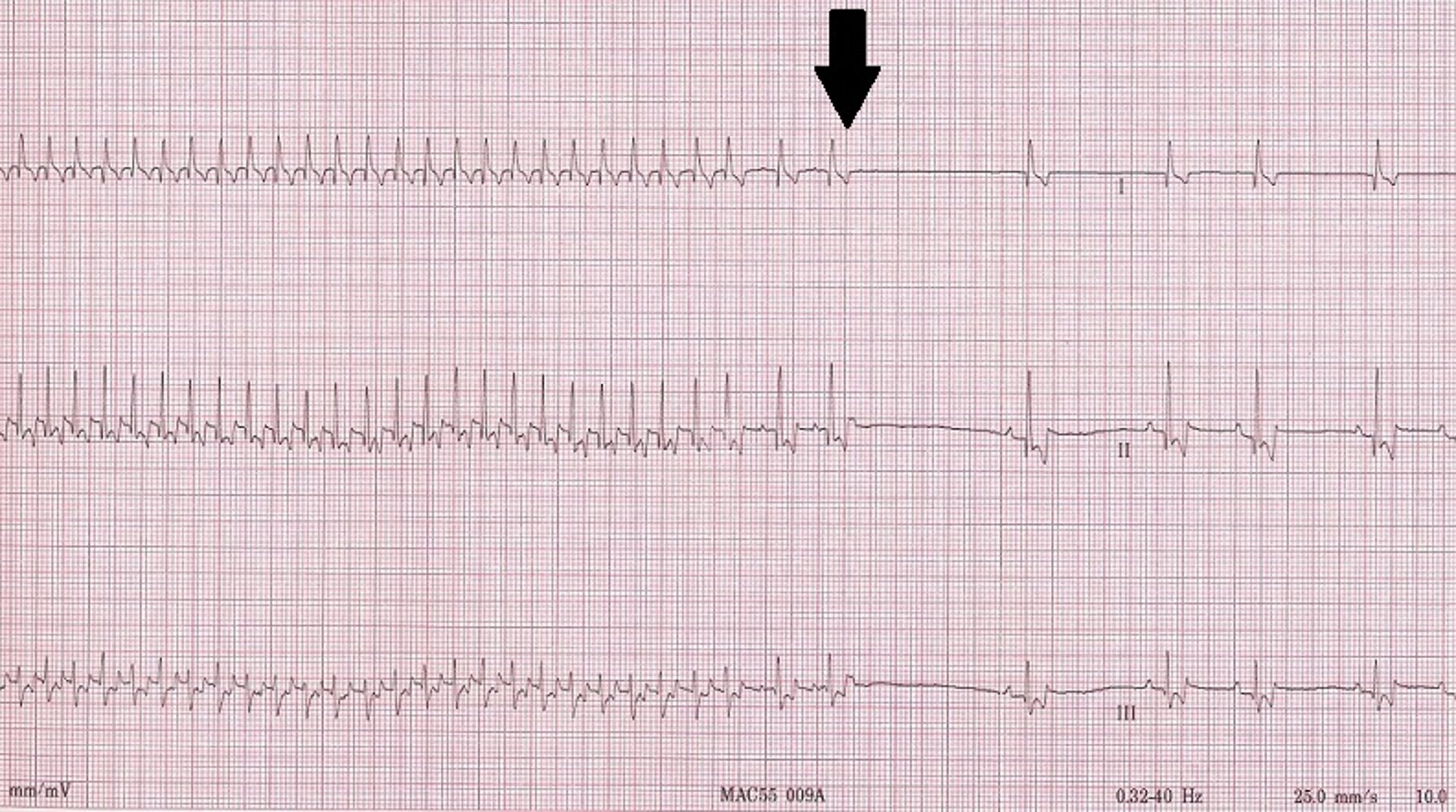
Sinus arrest (sinoatrial arrest, sinus pause) is the absence of P waves on the ECG for a short period (typically accepted as a pause exceeding twice the normal P-P interval). Sinus arrest results from excessive vagal tone, inherent sinus node disease, or both. Sinus arrest is usually due to some form of sick sinus syndrome (see below); however, it can sometimes occur with an exaggerated sinus arrhythmia.
Atrial standstill is characterized as the complete absence of P waves on the ECG and occurs when the atria are unable to be depolarized by the SA node discharge. Atrial standstill occurs either because the atrial myocardium is functionally unable to be depolarized (usually because of hyperkalemia), or because it has been destroyed by a cardiomyopathy or myocarditis (persistent atrial standstill). In hyperkalemia, the sinus node continues to depolarize, and the electrical tracts from the sinus node to the AV node (internodal tracts) continue to function, so the sinus node controls the rate (albeit at a lower rate). With persistent atrial standstill, the sinus node is destroyed, so the animal usually has an AV nodal (junctional) escape rhythm with a heart rate in the range of 40–65 bpm (dog).
Sick sinus syndrome is a constellation of abnormalities, including ECG changes (sinus arrest, junctional or ventricular escape complexes, and possibly supraventricular tachycardia) and possible weakness or syncope from the bradycardia (usual) or tachycardia (rare). With these clinical signs, the principal problem either lies within the SA node or perinodal tissue, or is due to increased vagal tone, or both. In some instances, other portions of the specialized conduction tissue of the myocardium, including the AV node, can also be affected. Therefore, evidence for AV block (see Atrioventricular Conduction Disturbances ) may also be present. This condition is most commonly noted in geriatric dogs, particularly in Miniature Schnauzers and Cocker Spaniels. Administration of parasympatholytics (eg, propantheline bromide, 0.25–0.5 mg/kg, PO, every 8–12 hours) or sympathomimetics (eg, extended-release theophylline, 10 mg/kg/day, PO; terbutaline, 0.2 mg/kg, PO, every 8–12 hours ) to increase heart rate can be tried.( 1 ) These are often effective; however, they may be effective for only a short time. Adverse effects can occur but are usually tolerable. Some dogs require pacemaker implantation.
Rishniw M, Thomas W. Bradyarrhythmias. In: Bonagura J, Kirk R, eds. Kirk’s current veterinary therapy: small animal practice. 13[ed.] ed. Philadelphia London: W.B. Saunders; 2000:719-725.
Atrioventricular Conduction Disturbances in Animals
The term atrioventricular (AV) block refers to an alteration of impulse conduction through the AV node from the atria to the ventricles. AV blocks are categorized as first-degree, second-degree, or third-degree blocks.
In first-degree AV block (prolonged conduction), the conduction time is increased and is recognized on an ECG as an increased P-R interval, with no attendant clinical signs
In second-degree AV block (intermittent conduction), occasional impulses fail to be conducted through the AV node, bundle of His, or both bundle branches; this type of block is most often characterized by occasional P waves that are not followed by QRS complexes. During the block, S 1 , S 2 , and arterial pulse are absent. S 4 may be audible in dogs with second-degree AV block, but it is much less common to hear S 4 in dogs. There are several different forms of second-degree AV block:
In Mobitz type I second-degree AV block , or Wenckebach phenomenon , the P-R intervals preceding the dropped beat progressively lengthen, or the P-R interval immediately after the block is shorter. This form of AV block is usually due to high vagal tone and is the most common type of second-degree AV block occurring in puppies. No treatment is indicated.
In Mobitz type II second-degree AV block , the P-R intervals do not change. Again, no treatment is indicated; however, closer surveillance may be warranted to see whether the block progresses to a more severe form.
High-grade second-degree AV block occurs with every other beat in a ratio of 2:1 (two P waves for every QRS complex) or more (3:1, 4:1, etc). This type of AV block is distinguished from third-degree AV block (see below) by the presence of an association between the QRS complexes and the P wave preceding each one (same P-R interval for each). Dogs with high-grade second-degree AV block can have clinical signs that match those of dogs with third-degree AV block (eg, syncope), and they are also at increased risk of sudden death.
In third-degree AV block (complete heart block), none of the impulses are conducted from the atria to the ventricles. The atrial rhythm (P waves) occurs more rapidly and independently from the ventricular rhythm (QRS complexes)—a form of AV dissociation. The ventricular rhythm originates from subsidiary pacemakers (AV node in the case of nodal escape beats, ventricular Purkinje fibers in the case of ventricular escape beats). The heart and pulse rates are usually regular but slow and generally unresponsive to factors or agents that usually increase heart rate (eg, exercise, excitement, atropine). The difference in timing between atrial and ventricular contractions results in variation in ventricular filling and consequent variation in the intensity of S 1 (bruit de canon) and possibly arterial pulse pressure. Periodically, the atria contract when the ventricle is in systole, resulting in a pulsation in the jugular vein (cannon A wave).
The importance of the type of AV block varies by species. Both first- and second-degree AV block may be present without outward evidence of cardiac disease. First-degree AV block may result from excessive vagal tone and usually is not important in dogs unless other evidence of heart disease or pathologic cause of increased vagal tone (eg, CNS or pulmonary disease) or AV nodal disease is present. In all species, second-degree AV block may indicate heart disease. Mobitz type II second-degree, high-grade second-degree, and third-degree (complete) AV blocks are always abnormal in all species.
Second- and third-degree AV blocks may be due to fibrosis, neoplasia, or injury to the AV node, or, rarely, to increased vagal tone or electrolyte abnormalities. The ideal treatment would be to correct the underlying cause, but this is not usually possible. High-grade second-degree AV block and third-degree AV block can cause exercise intolerance or, more commonly, weakness, collapse, and syncope. Oral treatment with positive chronotropic drugs, such as extended-release theophylline (10 mg/kg, PO, every 12 hours), terbutaline (0.2 mg/kg, PO, every 8–12 hours),( 1 ) or propantheline bromide (0.25–0.5 mg/kg, PO, every 8–12 hours) may occasionally be useful in animals with second-degree AV block; however, more aggressive treatment (pacemaker implantation) is usually indicated in symptomatic (eg, syncopal) animals.
Third-degree heart block is usually associated with irreversible lesions; the only effective treatment in dogs is pacemaker implantation. Because they are at risk of sudden death, dogs with third-degree AV block should have a pacemaker implanted regardless of clinical signs. In cats, third-degree AV block often produces no clinical signs, and thus requires no treatment. However, problems can arise if such a block is not identified before anesthesia, and some cats will faint and thus require pacemaker implantation. Pacemakers have been implanted successfully in species other than dogs and cats, but only rarely.
Common Tachyarrhythmias in Animals
Tachyarrhythmias can be categorized as supraventricular or ventricular on the basis of where they originate. Supraventricular premature complexes are premature complexes (as observed on an ECG) that originate from ectopic (nonautomatic) sites above the ventricles (eg, atrial myocardium or AV node). They may also be called atrial or nodal premature complexes/depolarizations/contractions/beats. Possible sites for ectopic depolarizations include the SA node (rare), atrial myocardium (very common), and AV node, or AV junction. On ECGs, supraventricular premature complexes are identified by a QRS complex that usually appears relatively normal but occurs earlier than the next expected normal QRS complex. Variable P wave morphologies may be noted before or after the supraventricular premature complex or may be hidden in the preceding sinus complex or within the premature complex. Supraventricular premature complexes are most commonly a result of atrial enlargement or disease, stress, or other causes of increased sympathetic tone.
Supraventricular tachycardia (SVT) is the consecutive occurrence of a series of supraventricular premature complexes. SVT may be short (nonsustained) or occur for prolonged periods (called "sustained" when lasting > 30 seconds). SVT most commonly ranges in rate from 200 to 350 bpm in dogs. At rates closer to 200 bpm, it may be indistinguishable from sinus tachycardia on a surface ECG. Vagal maneuvers (applying ocular pressure, carotid sinus massage), precordial blow (chest thump), and intravenous administration of drugs (eg, diltiazem) often "break" an SVT into sinus rhythm and either do not change or more gradually slow a sinus tachycardia. Diltiazem (dogs, 0.5–2 mg/kg, PO, every 8 hours; cats, 1.5–3 mg/kg or 7.5–15 mg/cat, PO, every 8 hours) is the most common drug used in longterm treatment of SVT; however, it can also be used intravenously to break the SVT into sinus rhythm (0.1–0.25 mg/kg, IV bolus over 5 minutes, followed 15 minutes later by up to 0.35 mg/kg over 5 minutes or constant rate infusion at dose rate of 0.05–0.15 mg/kg per hour). Digoxin and beta-blockers are also used.
An accessory pathway (bypass tract) is a congenital abnormality that forms an electrical connection between an atrium and a ventricle outside the normal connection (AV node/bundle of His). Accessory pathways have been recognized in dogs and cats and may result in SVT (eg, orthodromic AV reciprocating tachycardia). Treatment may include radiofrequency catheter (heat) ablation of the bypass tract or, more commonly, administration of oral medications such as procainamide, sotalol, or diltiazem.
Atrial flutter is a rare arrhythmia that often progresses to atrial fibrillation. It is most commonly due to a reentrant loop within the atria and is typically characterized on the ECG by a “saw-toothed” baseline with relatively normal QRS complexes that can appear in a regular or irregular rhythm. The atrial rate of discharge is very rapid (> 400 bpm). Only intermittent atrial impulses are conducted through the AV node, because of its normal long refractory period, so the ventricular rate is slower than the atrial rate.
In dogs and cats, atrial fibrillation is an even more rapid atrial rhythm (> 600–700 atrial depolarizations/minute) that results in a slower (in the range of 80–300 bpm in dogs) and always irregular ventricular rhythm. As in atrial flutter, the AV node is bombarded by frequent atrial depolarizations. The AV node acts as a filter, allowing only some of the depolarizations to reach the ventricles, but always in an irregular fashion. In dogs and cats, atrial fibrillation is characterized on the ECG by normal-appearing QRS complexes with an irregular ventricular rhythm that is usually fast (> 160 bpm). After those characteristics are identified, the next thing to look for is the absence of P waves and an undulating baseline that can appear almost flat (fine) or very rough (coarse). The irregular rhythm results in variation in the diastolic filling period of the ventricles and thus variability in stroke volume and variability in pulse character, including pulse deficits. The irregular rhythm also causes variation in the intensity of the heart sounds, especially the second heart sound, creating a heart sound that resembles "tennis shoes in a dryer" on auscultation in dogs.
In dogs, atrial fibrillation is most commonly associated with underlying cardiac disease. The notable exception occurs in some giant dog breeds, such as Irish Wolfhounds, Scottish Deerhounds, Great Danes, and others, in which the rhythm can develop with an otherwise normal heart (so-called lone or primary atrial fibrillation). All cats in atrial fibrillation have severe underlying heart disease.
The goal of treatment of atrial fibrillation in most dogs and all cats is to control the ventricular rate—ie, the frequency with which QRS complexes are generated from the fibrillatory depolarization waves. Rate control is usually accomplished by administration of either diltiazem (Dogs: diltiazem immediate release [0.5–2.0 mg/kg, PO, every 8 hours] or diltiazem extended release (XR) [3–5 mg/kg, PO, every 12 hours,]; Cats: diltiazem immediate release [3.75–7.5 mg/cat, PO, every 8 hours], or diltiazem controlled delivery (CD) [30–45 mg/cat, PO, every 24 hours], or diltiazem extended release (XR) [30–60 mg/cat, PO, every 12 hours]) or a combination of digoxin (0.003 mg/kg, PO, every 12 hours) and diltiazem. The combination is often more effective than diltiazem alone. A beta-blocker, such as atenolol, may also be used, but never in combination with diltiazem. These drugs prolong the refractory period of the AV node and slow AV nodal conduction, resulting in fewer atrial depolarizations crossing the AV node to the ventricles. Amiodarone has also been used to control the ventricular response rate; however, its adverse effects (hepatic and thyroid toxicoses) limit its use to second-line treatment in animals refractory to the digoxin and diltiazem/atenolol protocol.
In rare instances, electrical cardioversion (defibrillation of the heart that is synced to the ECG to prevent causing ventricular fibrillation) is used to convert atrial fibrillation to sinus rhythm. This method is most sensible in a dog with primary atrial fibrillation; however, it has also been done in dogs with atrial fibrillation secondary to severe cardiac disease. In those instances, sinus rhythm commonly reverts back to atrial fibrillation within weeks to months, necessitating reconversion or rate control. Cardioversion is frequently combined with amiodarone administration in an attempt to prolong the time until reversion to atrial fibrillation.
Ventricular premature complexes (VPCs) arise from a site within the ventricular myocardium or specialized intraventricular conduction system. On ECGs, the QRS complex usually appears wide and is followed by a large T wave that is opposite in polarity to the QRS complex. The result is a complex that is large and bizarre when compared with normally sinus-driven QRS complexes, occurs earlier than the next expected sinus-driven QRS complex (ie, it is premature), and does not have an associated preceding P wave, although unassociated P waves going at a lower rate (AV dissociation) may be observed. Most commonly, these complexes do not arise from primary cardiac disease, but instead result from systemic disturbances related to anesthesia, age, electrolyte abnormalities, acute toxicoses, neoplasia (eg, splenic hemangiosarcoma in dogs), gastric distention (eg, gastric dilation and volvulus syndrome in dogs), or trauma. They may also be associated with ventricular myocardial diseases such as dilated cardiomyopathy (DCM), arrhythmogenic right ventricular cardiomyopathy (ARVC; Boxer cardiomyopathy), and myocarditis .
Ventricular tachycardia is the occurrence of three or more sequential ventricular premature complexes. Again, these can be nonsustained or sustained (> 30 seconds). They can also be divided into slower, benign ventricular tachycardias and faster, malignant ones. A slower, benign ventricular tachycardia is called an accelerated idioventricular rhythm (AIVR). AIVRs occur commonly in dogs in the intensive care unit secondary to systemic (often intra-abdominal) disease or trauma. AIVRs are characterized on ECGs by the presence of a ventricular tachycardia that is relatively slow (usually
Fusion beats (hybrids of sinus beats and premature ventricular contraction [PVC]) can also occur. This arrhythmia does not result in sudden death and usually dissipates on its own within 48–72 hours. Therefore, it requires treatment (eg, lidocaine) only if it is causing hemodynamic instability.
Malignant ventricular tachycardia is most commonly the result of severe underlying cardiac disease, usually either a cardiomyopathy (eg, DCM or arrhythmogenic right ventricular cardiomyopathy ) or severe semilunar valve stenosis (eg, subaortic stenosis , pulmonic stenosis ). Malignant ventricular tachycardia predisposes the animal to sudden death because the tachycardia can deteriorate into ventricular fibrillation. Frequently, this arrhythmia is not identified, so the first clinical sign observed is sudden death. Some dogs (especially Boxers and Doberman Pinschers) will experience syncope as the result of a very fast ventricular tachycardia (often > 400 bpm) that spontaneously reverts back to sinus rhythm within seconds of its onset (ventricular tachycardia by definition must last > 6 seconds and usually lasts no more than 1 minute).
Administration of sotalol or a combination of atenolol and mexiletine effectively controls the arrhythmia in Boxers and usually stops the syncope and presumably prevents sudden death. Beta-blockers are frequently administered to dogs with severe subaortic stenosis and to some with severe pulmonic stenosis in an attempt to prevent sudden death, but proof of efficacy is lacking. Ventricular tachycardia must be distinguished from ventricular escape rhythm, as observed with third-degree AV block, and from idioventricular rhythm, a terminal ventricular escape rhythm. A ventricular escape rhythm is a slow rhythm (20–40 bpm) that occurs because higher pacemakers (SA and AV nodes) have failed. Suppression of a ventricular escape rhythm by administration of a drug (eg, lidocaine) results in cessation of all cardiac electrical activity (ie, death).
Ventricular fibrillation is a result of microreentrant circuits within the ventricular myocardium, resulting in the absence of effective ventricular contractions; thus, it is a terminal rhythm (ie, cardiac arrest). The only effective treatment is electrical defibrillation.
Antiarrhythmics for Animals
A detailed discussion of antiarrhythmic treatment is covered elsewhere (see Antiarrhythmics in the chapter "Systemic Pharmacotherapeutics of the Cardiovascular System"). Most antiarrhythmic drugs are administered to suppress ectopic premature depolarizations (eg, atrial and ventricular premature complexes, atrial and ventricular tachycardia) or to slow the ventricular rate in animals with atrial flutter or fibrillation. Many antiarrhythmics are being supplanted by automatic implantable defibrillators in human medicine, so the manufacture of these drugs is waning. Some antiarrhythmics have negative inotropic effects, with the potential to worsen active CHF; this is most likely to occur with the use of beta-blockers in the treatment of supraventricular tachyarrhythmias and with sotalol.
Atrial fibrillation is one of the most commonly treated tachyarrhythmias ; it is imperative to decrease the ventricular rate to ≤160 bpm if it is higher than that in the clinic. In experimental situations, pacing the heart of a dog at a rate ≥180 bpm results in myocardial failure severe enough to cause CHF within weeks. Consequently, leaving the rate this high will cause further cardiac disease and decompensation. The target heart rate is controversial, but an average (mean) heart rate of Antiarrhythmics ).
Ventricular tachycardia can degenerate into ventricular fibrillation and cause sudden death. Dogs with fast ventricular tachycardia (> 250 bpm) or with ventricular tachycardia accompanied by severe underlying cardiac disease are the most vulnerable to dying suddenly from ventricular tachycardia. In Boxers with ARVC, administration of sotalol (0.5–3 mg/kg, PO, every 12 hours; most commonly 80 mg/dog, PO, every 12 hours) or of a combination of mexiletine (5–8 mg/kg, PO, every 8 hours) and either atenolol (0.5–1 mg/kg, PO, every 12 hours) or sotalol can effectively decrease or, more commonly, stop episodes of syncope due to ventricular tachycardia and decreases the incidence of sudden death. In addition, Doberman Pinschers with DCM commonly die suddenly as a result of ventricular tachycardia. Caution is warranted when treating patients with DCM with sotalol, as the negative inotropic effects of this drug can either push a dog into heart failure or make existing heart failure worse. Consequently, in these cases, the dosage must start low and be titrated upward carefully, with pimobendan administered concurrently. Amiodarone (loading dose: 8–10 mg/kg, PO, every 12 hours for 7–10 days; maintenance dose: 4–6 mg/kg, PO, every 24 hours) is another treatment option for preventing sudden death, but it has frequent adverse effects. Doberman Pinschers appear to be particularly susceptible to the hepatotoxic effects of amiodarone.
Animals with chronic bradyarrhythmias, as occur with AV block (high-grade second-degree block or third-degree block) or sick sinus syndrome, most commonly present with weakness, episodic weakness/collapse, and syncope. Pacemaker implantation is the treatment of choice. If pacemaker implantation is not a viable option, anticholinergics, phosphodiesterase (PDE) inhibitors, or sympathomimetics may be administered.
Propantheline is a mild anticholinergic dosed as follows: for dogs, 0.25–1 mg/kg, PO, every 8–12 hours; for cats, 0.8–1.6 mg/kg or 7.5 mg/cat, PO, every 8–12 hours for maximum 3 days. The parenteral formulation of atropine may be administered orally, but it must be diluted 10:1 with corn syrup at a dosage of 0.04 mg/kg, PO, every 6–8 hours. Adverse effects include mydriasis, dry mucous membranes, tachycardia, and GI stasis.
Theophylline is a nonselective PDE inhibitor with modest positive chronotropic effects. Extended-release tablets or capsules can be given at 10 mg/kg, PO, every 12 hours; consider monitoring. If no adverse effects are evident and the desired clinical effect is not achieved, the dosage in dogs can be increased to 15 mg/kg, PO, every 12 hours, while monitoring for adverse effects; and in cats, to 20 mg/kg, PO, every 24–48 hours. Adverse effects may include restlessness, excitability, tachycardia, or GI upset.
Terbutaline is a beta-agonist that has more potent positive chronotropic effects; however, its adverse effects are similar to those observed with theophylline. It is dosed to effect at 1.25–5 mg/dog (not per kg), PO, every 8 hours; and 0.625 mg/cat, PO, every 12 hours.
Oral treatment of clinically important bradyarrhythmias that are due to high-grade second-degree or third-degree AV block is often unsuccessful, although overall clinical signs may improve in some animals. Sick sinus syndrome is more often amenable to medical treatment.

Identifying Arrhythmias in Veterinary Patients
Amara h. estrada , dvm, dacvim (cardiology), university of florida.

Successful identification of an arrhythmia requires understanding the cardiac conduction system.
A normal working rhythm includes the following:
Heart rate in a normal or expected range for the breed, species, and clinical situation (eg, sinus arrhythmia and/or wandering pacemaker are considered normal in a relaxed dog but are not normal in a cat being examined in a clinical setting; Figures 1-3 )
Rhythm in which there is a P wave for each QRS complex, a QRS complex for each P wave, and (most importantly) a relationship between the P waves and QRS complexes
Constant atrioventricular (AV) interval
QRS complex that appears upright and narrow in leads II, III, and aV
P wave that appears upright in leads I, II, III, and aVF.

ECG showing a normal sinus rhythm

ECG showing a sinus arrhythmia with a regularly irregular rhythm, in which the heart rate increases and decreases in a pattern. This is considered normal in relaxed dogs.

ECG and illustrations showing a wandering pacemaker. Tall P waves with fast heart rates and high sympathetic tone and short P waves with slow heart rates and high vagal tone can be seen. This is considered normal in relaxed dogs.
Related article: Interpreting ECGs with Confidence
Clinical Response to an Abnormal Rhythm
In patients with an abnormal ECG, whether the arrhythmia is hemodynamically important (eg, affecting cardiac output, in a breed with known risk for sudden death) should initially be determined. An arrhythmia that has not resulted in cardiac enlargement is likely not hemodynamically affecting the patient but may indicate presence of underlying heart disease, warranting echocardiographic evaluation.
Bundle Branch Block
The ECG hallmark of a bundle branch block is a markedly widened QRS complex (dogs, ≥80 ms in duration on ECG; cats, >40 ms in duration on ECG). Although these blocks have a unique pattern of conduction, they are supraventricular in origin (ie, sinus rhythm in which the impulse has been initiated from the SA node and conduction is through the AV node but with a different pattern) and should not be misinterpreted as ventricular tachycardia (VT). Identification can be accomplished by noting a P wave associated with the QRS complex and a constant PR interval ( Figure 4 ).

Illustration and representative ECGs of a right bundle branch block (RBBB) and a left bundle branch block (LBBB). QRS complexes are wide in both blocks, but a P wave is present for each QRS complex, a QRS complex is present for each P wave, and the PR interval is constant. With an RBBB, impulse formation travels down the left side normally, but the right ventricle is delayed in depolarization because it has to use slow muscle cell to muscle cell conduction; depolarization and repolarization of the right ventricle therefore take longer than normal (≥80 ms measured on ECG). Impulse formation is complete after the right ventricle is depolarized by the impulse moving from the apex toward the base of the right side of the heart, creating a right axis shift and an ECG with deep S waves in leads II, III, and aVF but a constant PR interval and normal heart rate. With an LBBB, there is no axis shift because ventricular depolarization typically occurs toward the left apex. A markedly prolonged QRS complex, normal and constant PR interval, normal mean electrical axis, and normal heart rate are seen instead.
Atrioventricular Nodal Block
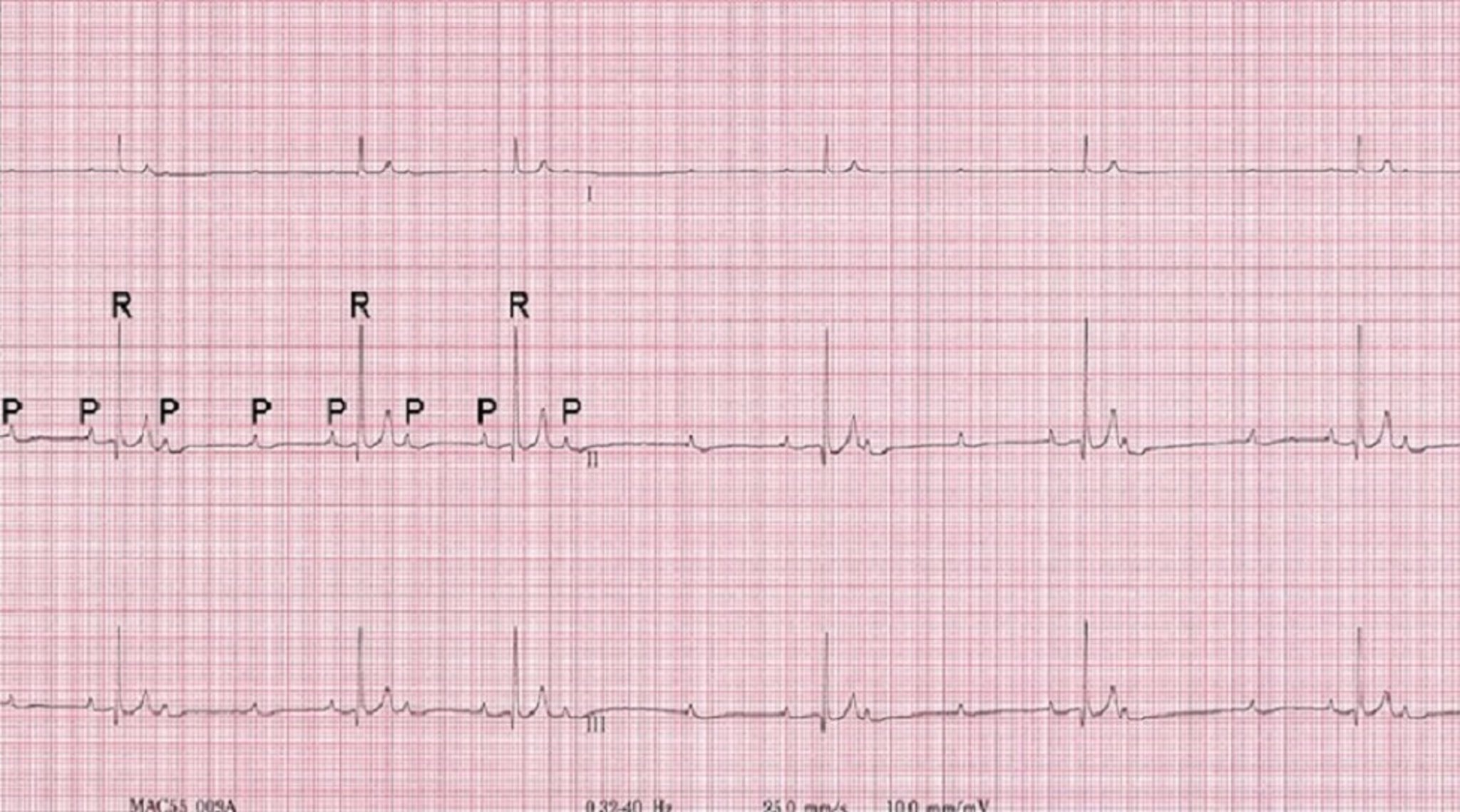
Evaluation of an arrhythmia with AV nodal disease includes determination of whether the arrhythmia is hemodynamically important (ie, rhythm is slow enough to cause clinical signs, including exercise intolerance, syncope, and signs of congestive heart failure) and whether there is autonomic influence on the arrhythmia that can be accomplished with administration of a high-end dose of atropine (0.04 mg/kg SC or IM). Expected response for a normal sinus or AV node with excessive vagal influence is complete resolution of the AV block and a heart rate >160 to 180 bpm ( Figure 5 ).
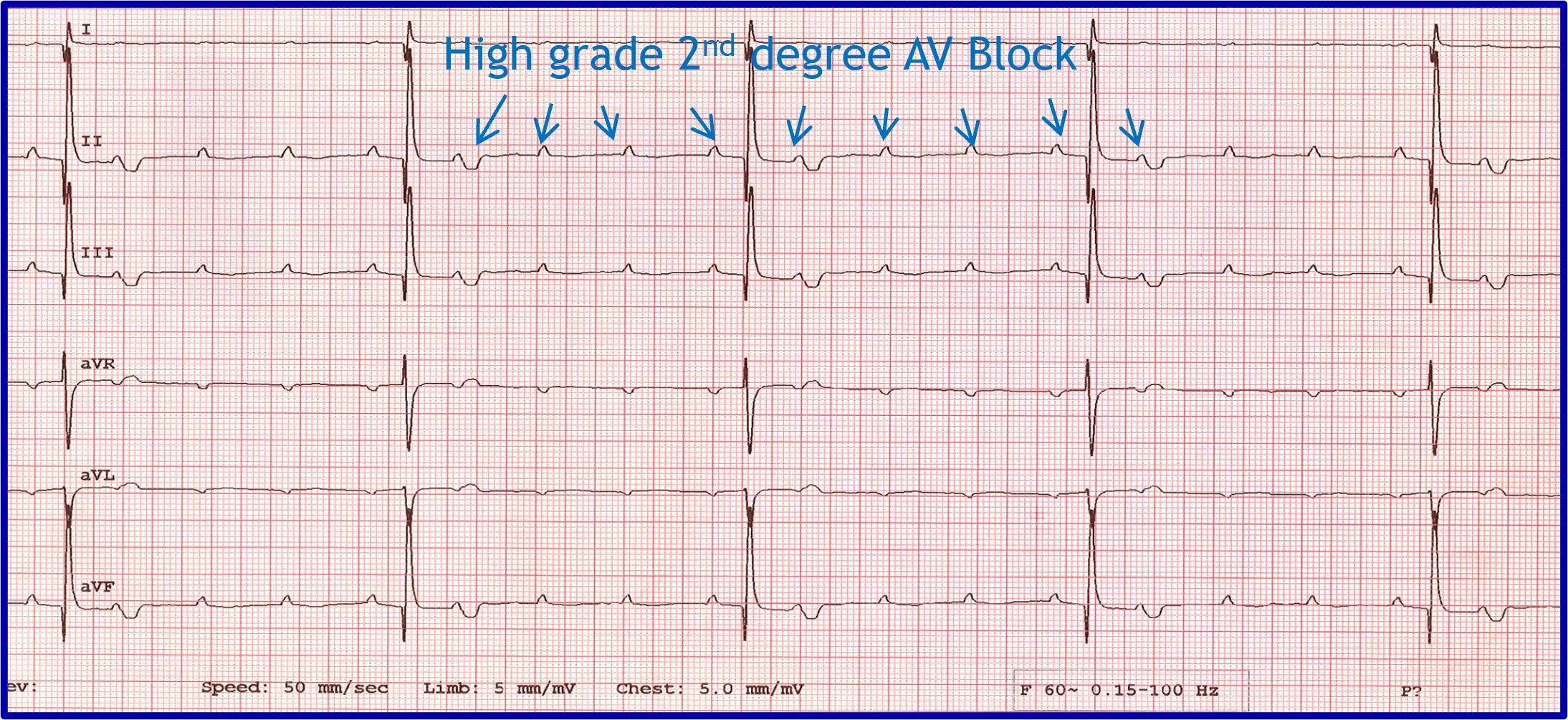
( A ) ECG in a patient with high-grade second-degree AV block in which no 2 consecutive P waves are conducted to create ventricular depolarization. Each QRS complex has a P wave with a constant PR interval, but many P waves do not have an accompanying QRS complex. ( B ) Thirty minutes after administration of atropine (0.04 mg/kg IM), ECG showed complete resolution of the AV block, 1:1 conduction of every P wave, and heart rate >180 bpm, indicating the SA and AV nodes are fully functional and the prior delay in conduction was vagally mediated.
Premature Beats
Premature beats occur abnormally fast compared with the previous beat and other prior beats and can be individual beats, couplets, triplets, or runs of tachycardia. Evaluation should include assessment of where the premature beats originated and how they traveled within the heart.
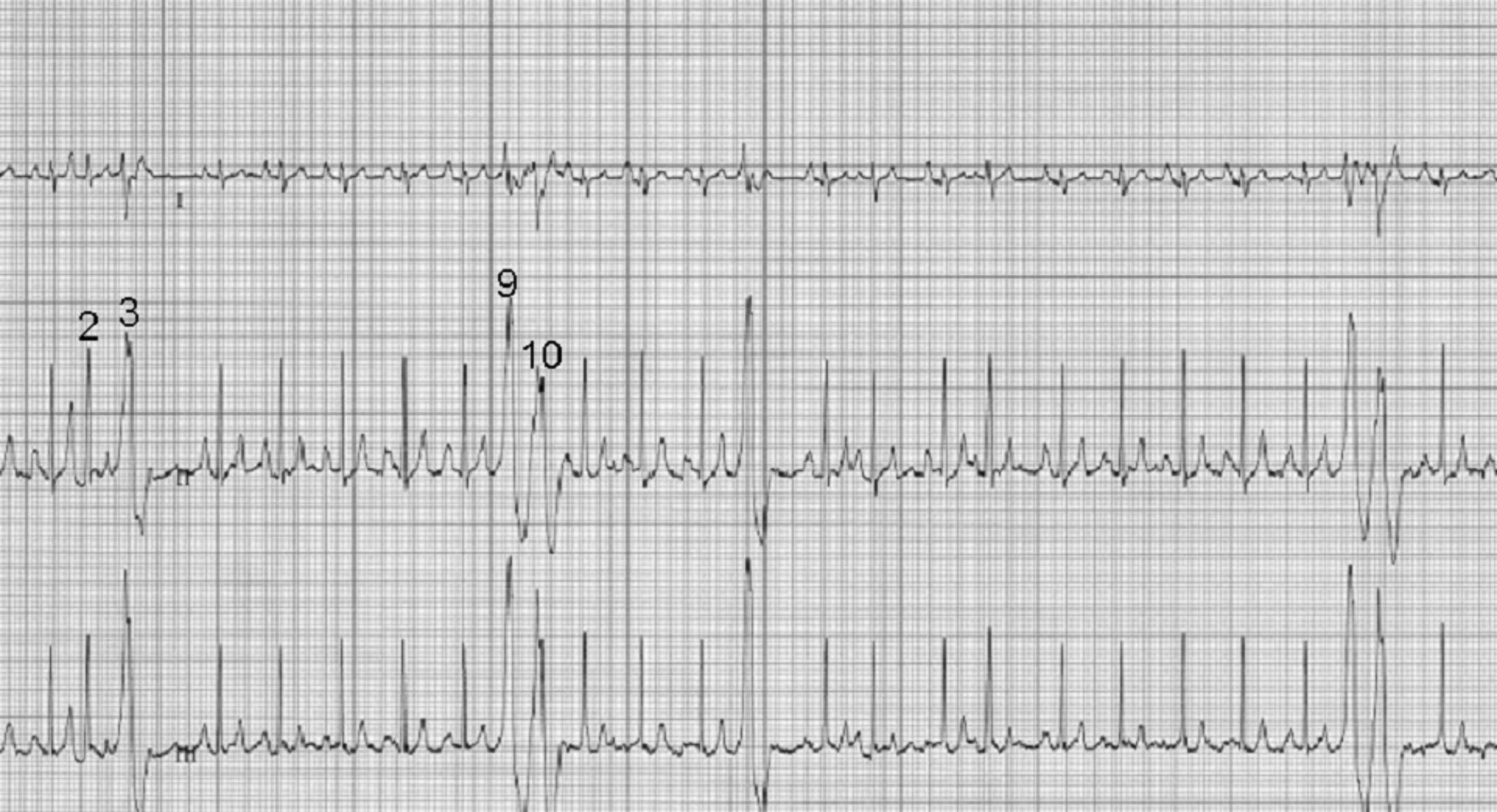
Premature beats originating at or above the AV junction can still be rapidly conducted to the ventricular myocardium via specialized conduction tissue and therefore appear nearly identical to the regular rhythm. Premature beats originating below the AV junction (ie, below the bundle of His) cannot use the specialized conduction system and must therefore depolarize the ventricles via muscle cell to muscle cell conduction. This is a relatively slow process that produces a wide, bizarre QRS complex that can be predominantly positive or negative depending on where and in which ventricle the impulse starts and what direction it travels from to allow for complete ventricular depolarization. There will not be a related P wave because the impulse forms and depolarizes independently from atrial depolarization. Because depolarization is abnormal, repolarization is also abnormal and is represented by a large, bizarre T wave on ECG ( Figure 6 ).
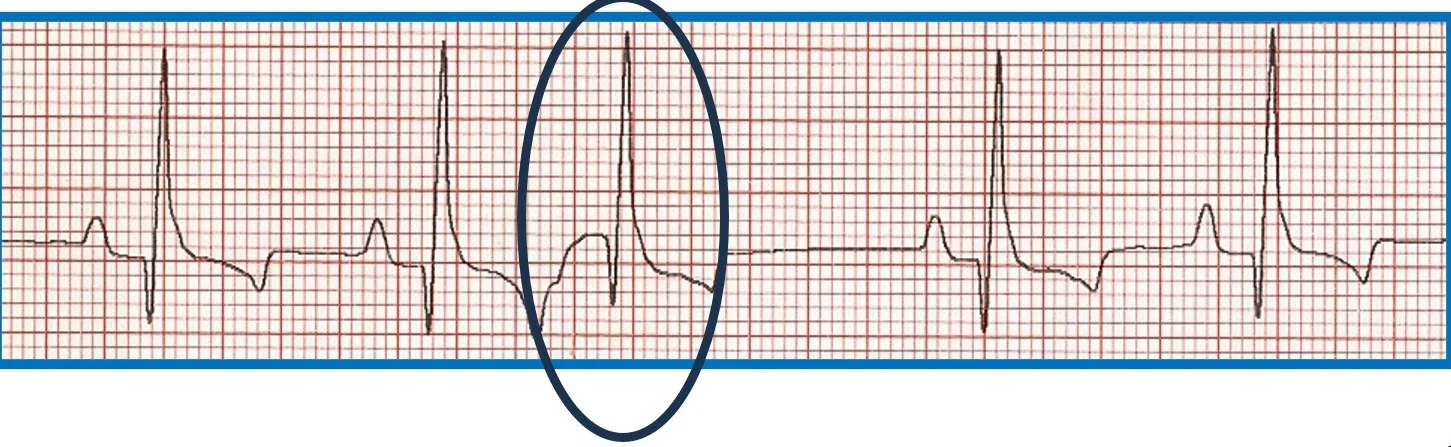
FIGURE 6 Effect of ectopic foci origination location on ECG appearance. ( A ) ECG showing a normal QRS complex but indiscernible P wave ( circle ) characteristic of premature beats with supraventricular origin; the circled premature beat is almost identical to the beats with an associated P wave. ( B ) Illustration demonstrating where ectopic foci result in supraventricular ( above dotted line ) and ventricular ( below dotted line ) ECG patterns. ( C ) ECG showing no P wave; a wide, bizarre QRS complex; and a large, bizarre T wave ( circles ) characteristic of premature beats with a ventricular origin.
Accelerated Idioventricular Rhythm
Runs of ventricular ectopy are not always dangerous. Many systemic diseases can cause accelerated idioventricular rhythm (AIVR; ie, slow VT; Figure 7 ). Rhythm rate is key for distinguishing AIVR from dangerous VT. AIVR rate is typically <180 to 200 bpm, and VT is typically >250 bpm. Couplets, triplets, or runs with a rate between 200 and 250 bpm indicate a possible need for therapy in patients with no concurrent systemic or underlying cardiac disease that could account for the rhythm disturbance.

ECG showing an AIVR rhythm in a Lhasa apso with immune-mediated hemolytic anemia
Sick Sinus Syndrome/Sinus Node Dysfunction
Sick sinus syndrome (SSS)/sinus node dysfunction (SND) is a conduction disease of the SA node believed to be degenerative. Other regions of the conduction system may also be diseased. Escape rhythms do not always, therefore, appropriately rescue the rhythm. ECG features of SSS/SND include sinus arrest (ie, cessation of SA node activity/no atrial activity or P waves identified and absence or delay of expected escape/rescue rhythms from other automatic tissues in the heart [eg, bundle of His, Purkinje fibers, ventricular tissue]; Figure 8 ). For example, the intrinsic rate of the AV node is 40 to 60 bpm. If the SA node pauses >1.2 seconds, the AV junction should depolarize and produce a junctional beat. Likewise, if the SA node (intrinsic rate, 30 bpm) pauses for >2 seconds, the Purkinje fibers should fire and rescue the rhythm; however, this often does not occur or is inappropriately delayed in patients with SSS/SND, and sinus arrest/asystole can last for several seconds. Junctional rhythms may become the dominant rhythm. Administration of a high-end dose of atropine (0.04 mg/kg SC or IM) may result in an increase in heart rate. Lack of an appropriate response (ie, AV block resolves, sinus rate increases to >180 bpm, every P wave is associated with a QRS complex, PR intervals are constant) is diagnostic for SSS/SND; however, a partial or even complete response does not necessarily rule out SSS/SND. SSS/SND is highly influenced by autonomic tone; therefore, a partial or full response to atropine in a breed predisposed to SSS/SND (eg, West Highland white terriers, schnauzers, dachshunds, cocker spaniels) with clinical signs and ECG or Holter characteristics of SSS/SND does not exclude this diagnosis.
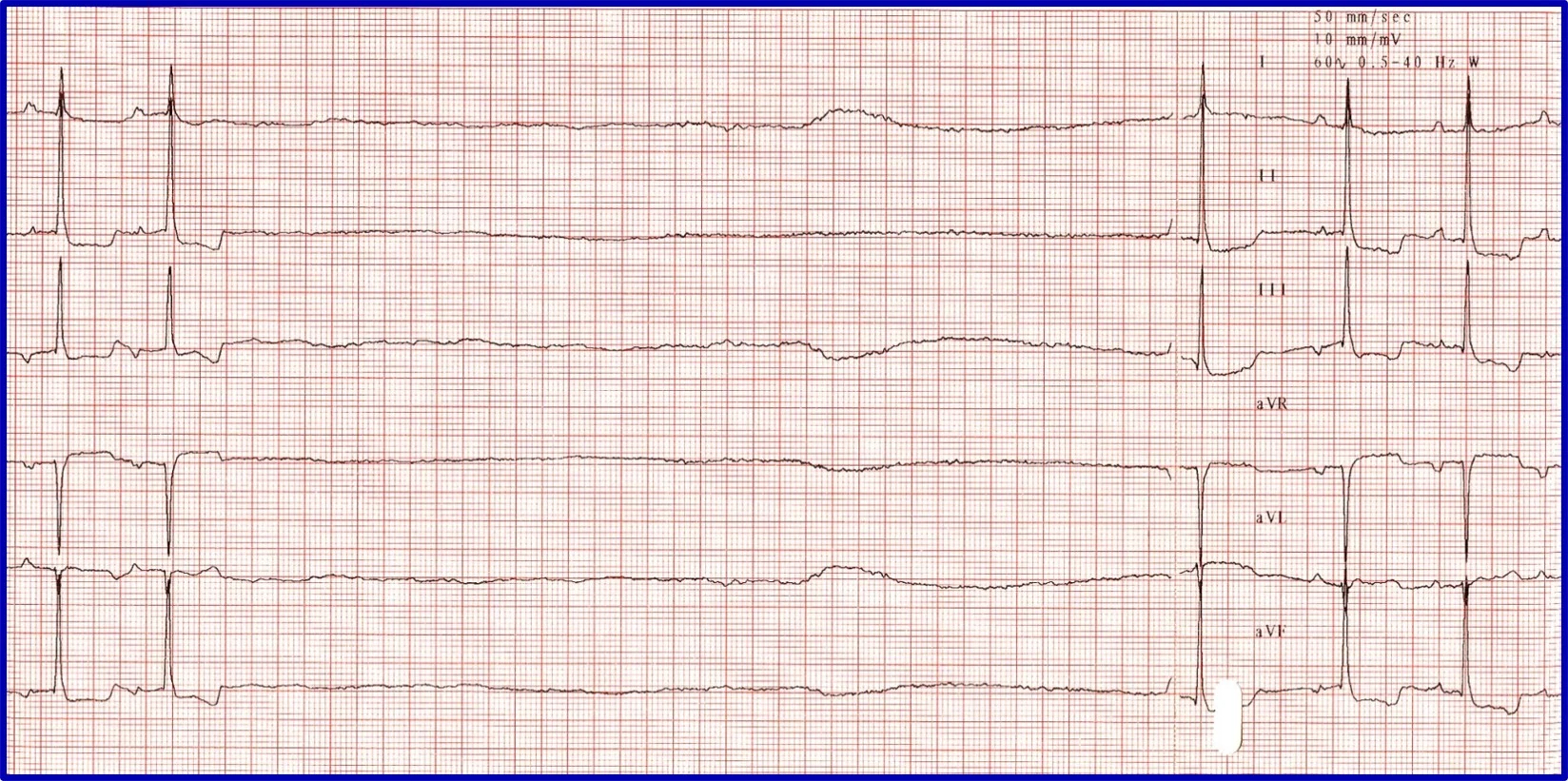
( A ) ECG in a patient with SSS/SND and a delay of escape focus to rescue the rhythm. ( B ) Thirty minutes after administration of atropine (0.04 mg/kg IM), ECG showed resolution of SSS/SND, but the SA node was only able to speed up to 140 bpm, indicating that although the dysfunction was heavily influenced by autonomic tone, there was an incomplete response to obliterating vagal tone. The SA node was not able to operate at full function.
Atrial Fibrillation
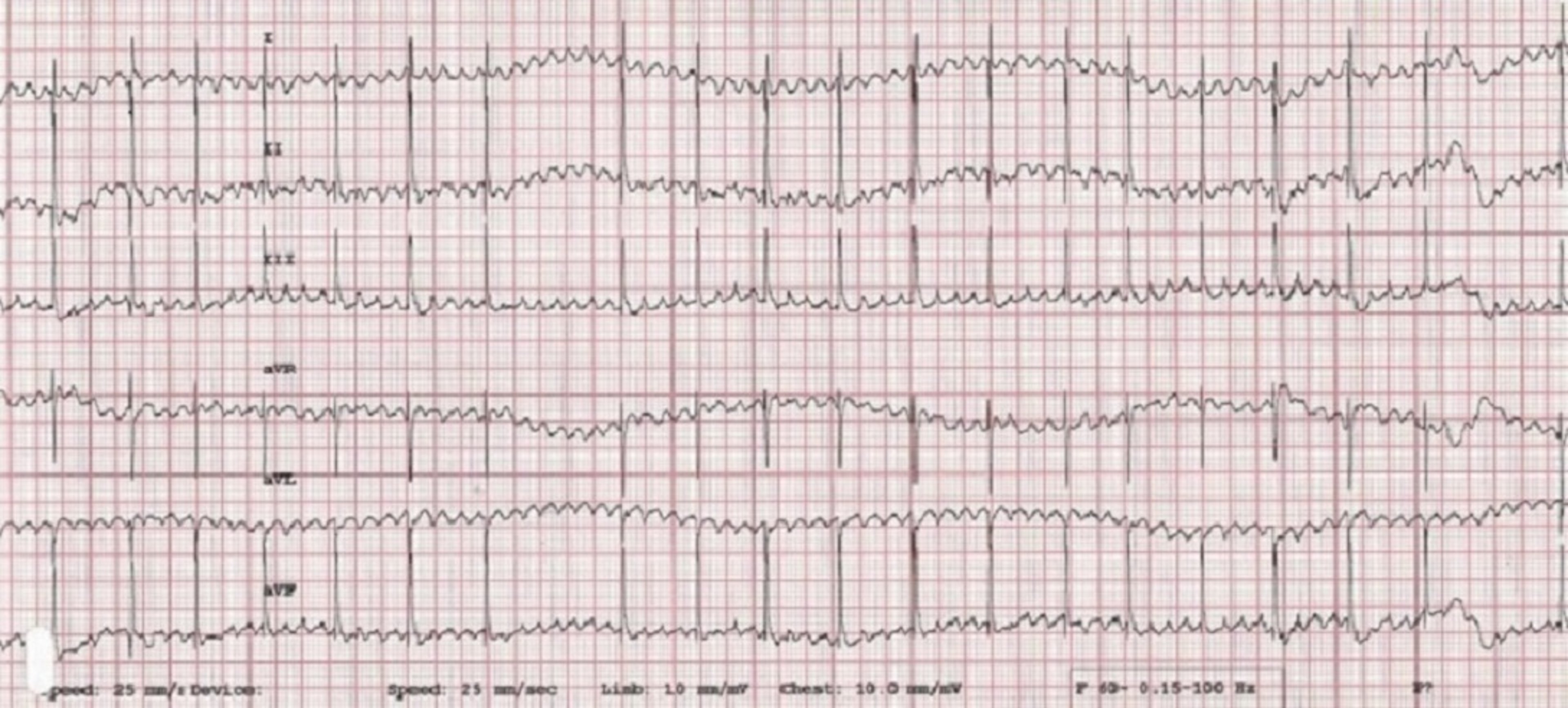
ECG features of atrial fibrillation (AF) include an irregularly irregular rhythm (variable RR intervals) with no identifiable pattern. P waves cannot be reliably identified, and heart rate is usually >200 bpm. QRS complexes typically appear to be supraventricular (ie, narrow and upright in lead II); however, some deep-chested breeds (eg, Doberman pinschers, Irish wolfhounds) develop wider QRS complexes with left ventricular enlargement instead of a tall QRS. The baseline may have irregular undulations (ie, fibrillation waves), but this is not a requirement for diagnosis. Fibrillation waves are not always present or identifiable, especially when the heart rate is fast. Aberrantly conducted beats may cause variation in the height and morphology of QRS complexes ( Figure 9 ).

ECG in a patient with AF and aberrant ventricular conduction. A fusion beat (ie, simultaneous ventricular premature complex and normal sinus beat that results in an ECG trace that is a sum of the 2 vectors of depolarization) can be seen ( circle ). With AF, the atria fibrillate at 500 to 600 bpm; the AV node cannot discern whether it should conduct impulses and thus attempts to conduct all impulses but is unable to do so because it cannot depolarize and repolarize at that rate. Beats that do not appear supraventricular in origin were conducted when only parts of the conduction system were repolarized and ready to conduct an impulse from the AV node to the ventricular myocardium; these impulses take an abnormal pathway to the ventricle, resulting in the appearance of a wide, bizarre ventricular beat ( arrows ). For example, if the right bundle branch is repolarized and ready to conduct while the left bundle is still in a refractory period, an ECG complex that resembles an LBBB occurs. A different conduction pattern is possible with almost every beat, as the right and left bundles can be at different phases of refractoriness when each impulse is presented. This type of aberrant conduction is common with AF and important to recognize because it is not a dangerous ventricular rhythm and should not be treated with lidocaine or other ventricular antiarrhythmic therapy.
Irregularity can be difficult to identify when AF rate is >250 bpm. Multiple ECGs or ECGs with multiple leads (eg, 6- or 12-lead ECG) are needed. ECG recorded at a faster speed (eg, 50 mm/second) can also be helpful. A rapid, irregular supraventricular tachycardia without identifiable P waves should be considered AF until proven otherwise. If an alternative diagnosis is possible, AF should be ruled out first, as it is the most likely possibility.
Ventricular Arrhythmia
Although identifying ventricular arrhythmias (ie, malignant ventricular premature complexes, VT) is not difficult, deciding whether and when to treat a ventricular ectopic rhythm can be challenging.
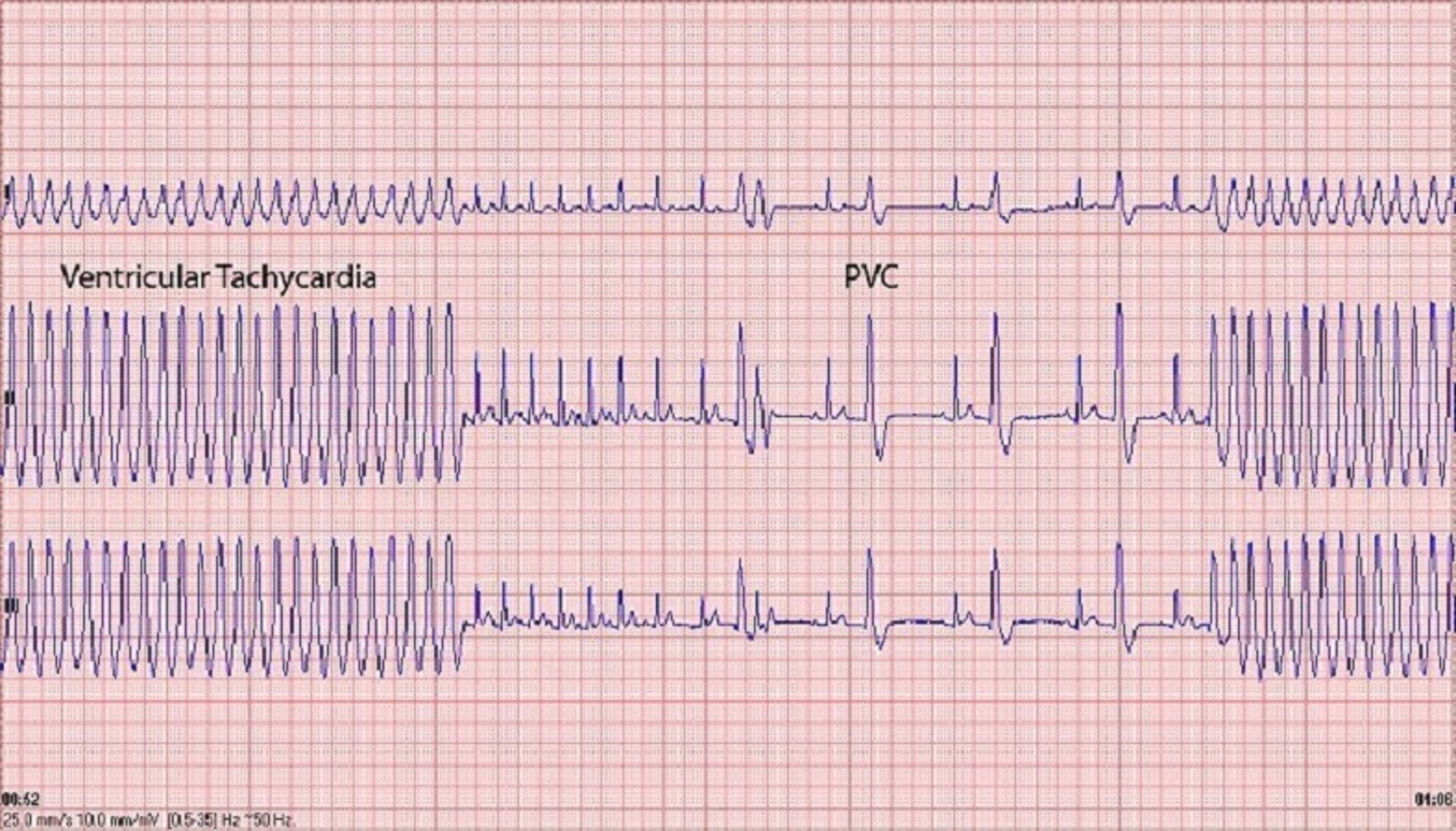
It is important to discern whether the arrhythmia is causing hemodynamic compromise by evaluating peripheral pulse quality, mucous membrane color, patient behavior, and arterial blood pressure. Sustained VT at a high rate is more likely to cause hemodynamic compromise than VT at a slower rate or AIVR ( Figure 10 ). Determining whether the arrhythmia is likely to degenerate into ventricular fibrillation, which causes sudden death, is also important. Increased speed of VT or ventricular ectopic beats increase the likelihood that a beat will fall within the vulnerable period and induce ventricular fibrillation (ie, R on T phenomenon).
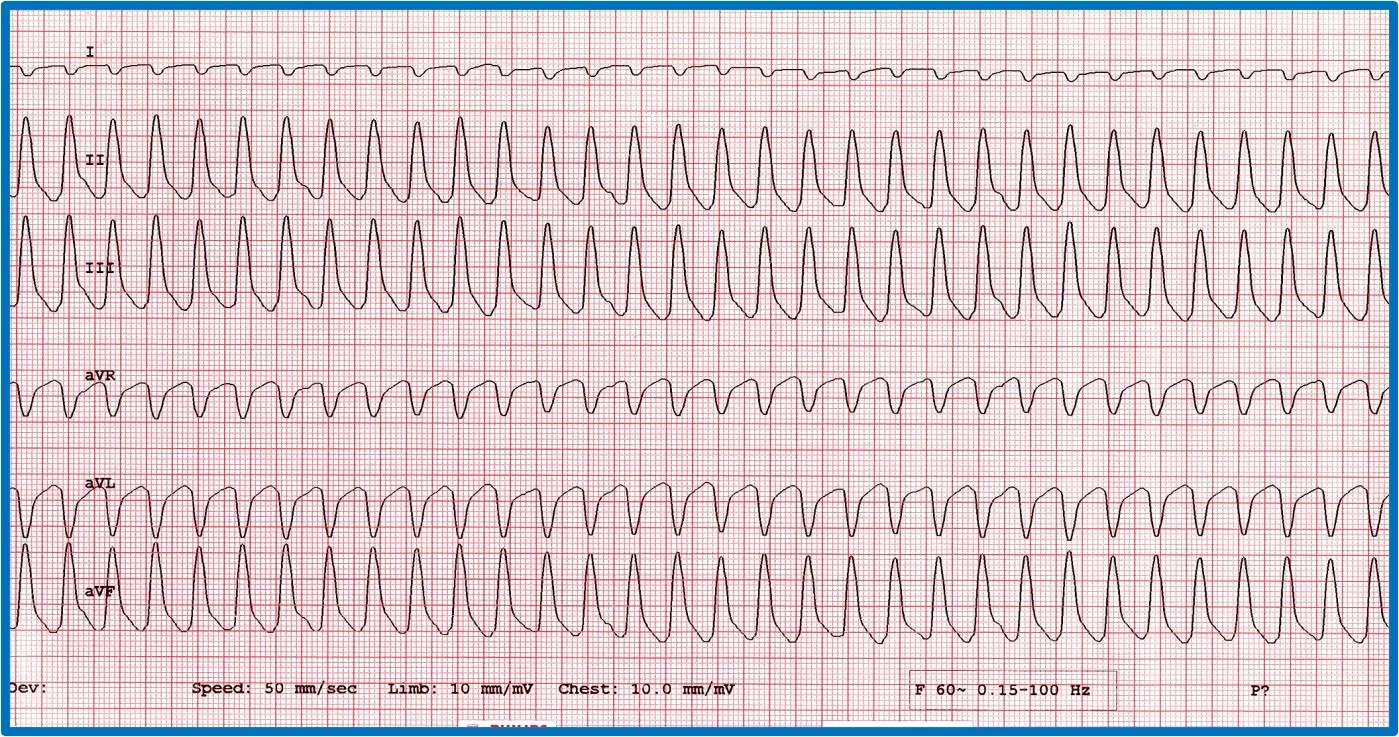
Two versions of ventricular runs of ectopy. ( A ) ECG showing a run of sustained true VT with a rate of 320 bpm in a boxer. ( B ) ECG showing an AIVR rhythm (ie, slow VT) in a crossbreed dog hit by a car and experiencing traumatic myocarditis. The rate is 180 bpm and not hemodynamically compromising the patient. A sinus beat occuring at the same time as a ventricular beat, causing a fusion beat ( circle ), can be seen.
Polymorphic VT, ventricular premature complexes that occur in couplets at a fast coupling rate, and ventricular ectopy are thought to be more dangerous than monomorphic VT or ventricular ectopy. Certain patient groups (eg, Doberman pinschers with dilated cardiomyopathy , boxers with arrhythmogenic right ventricular cardiomyopathy, patients with severe subaortic stenosis, German shepherd dogs with inherited ventricular arrhythmias, cats with hypertrophic cardiomyopathy) may have an increased risk for sudden death associated with VT and ventricular ectopy; treatment of ventricular arrhythmias in these patients is therefore typically recommended.
Connect with Us
Veterian Key
Fastest veterinary medicine insight engine.
- ANIMAL RADIOLOGY
- EQUINE MEDICINE
- EXOTIC, WILD, ZOO
- FARM ANIMAL
- INTERNAL MEDICINE
- NURSING & ANIMAL CARE
- PHARMACOLOGY, TOXICOLOGY & THERAPEUTICS
- SMALL ANIMAL
- SUGERY, ORTHOPEDICS & ANESTHESIA
- Abdominal Key
- Anesthesia Key
- Basicmedical Key
- Otolaryngology & Ophthalmology
- Musculoskeletal Key
- Obstetric, Gynecology and Pediatric
- Oncology & Hematology
- Plastic Surgery & Dermatology
- Clinical Dentistry
- Radiology Key
- Thoracic Key
- Veterinary Medicine
- Gold Membership
Disorders of Cardiac Rhythm
Table 145-3 COMMON ANTIARRHYTHMIC DRUGS: FORMULATIONS, INDICATIONS, AND DOSAGES Chapter 145 Disorders of Cardiac Rhythm Michael S. Miller, Larry Patrick Tilley, Francis W.K. Smith, Jr. Cardiac arrhythmias include disorders of cardiac impulse formation, conduction, rate, and regularity. Terms such as dysrhythmia, ectopia, and ectopy also are used to identify arrhythmias. Cardiac arrhythmias can be benign and clinically insignificant, or they can cause clinical signs. They can even progress to malignant arrhythmias that lead to heart failure, syncope, or sudden death. Causes of cardiac arrhythmias include heart disease and disorders involving the autonomic nervous system, endocrine system, electrolytes, and other body systems. Anesthetic agents and other drugs can precipitate rhythm disturbances. Cardiac arrhythmias are diagnosed and classified electrocardiographically; see Chapter 144 for additional pertinent information regarding electrocardiography. A summary of the clinical pharmacology of drugs used in the treatment of congestive heart failure (CHF) is found in Chapter 146 . ETIOLOGY • Cardiac arrhythmias are classified in Table 145-1 . They occur with congenital or acquired cardiac disease or systemic disorders ( Table 145-2 ). • Cardiac pathology does not necessarily correlate with the type and severity of arrhythmias. • Arrhythmia variation in animals with cardiac or systemic disorders may be explained by the complex interactions among cardiac cell transmembrane potentials, the autonomic nervous system, and body fluids. Table 145-1 CLASSIFICATION OF CARDIAC ARRHYTHMIAS Supraventricular Rhythms Sinus rhythm Sinus arrhythmia Sinus bradycardia Sinus tachycardia Atrial premature complexes Sinus block and/or arrest Atrial tachycardia Atrial/supraventricular tachycardia (reentrant) Atrial flutter Atrial fibrillation Atrioventricular junctional rhythm Ventricular Rhythms Ventricular escape (rhythm) Ventricular premature complexes Idioventricular tachycardia Ventricular tachycardia Ventricular asystole Ventricular fibrillation Conduction Disorders Atrial standstill First-degree AV block Second-degree AV block Complete (third-degree) AV block Arrhythmias and Conduction Disturbances Sick sinus syndrome Ventricular preexicitation and the Wolff-Parkinson-White syndrome Table 145-2 CAUSES OF CARDIAC ARRHYTHMIAS Adapted from Miller MS, Tilley LP: Treatment of arrhythmias and conduction disturbances. In Miller MS, Tilley LP, eds.: Manual of Canine and Feline Cardiology. Philadelphia: WB Saunders, 1995, with permission. Cardiac Causes in Dogs Heredity (genetics not documented in all cases) Doberman (His bundle degeneration) English springer spaniel (persistent atrial standstill) Miniature schnauzer, dachshund, cocker spaniel, West Highland white terrier (sick sinus syndrome) Pug, Dalmatian (sinus node disease) Pug (stenosis and degeneration of the His bundle) Wolff-Parkinson-White syndrome Golden retriever (Duchenne muscular dystrophy) German shepherd (ventricular tachyarrhythmia) Atrial and/or ventricular arrhythmias Atrial enlargement, secondary to congenital defects or acquired disease Cardiomyopathy Congenital heart disease Congestive heart failure Mitral valve disease (congenital and acquired) Myocarditis, endocarditis Myocardial ischemia Trauma Drugs Conduction system disease Acquired sinus and AV node disease (sick sinus syndrome) Cardiomyopathy Neoplasia Surgical damage to conduction tissue Trauma Vascular (e.g., microscopic intramural myocardial infarction) Ventricular septal defect and other congenital defects Infection (Lyme disease) Drugs Degeneration Noncardiac Causes in Dogs and Cats Heredity (rare) Wolff-Parkinson-White syndrome Atrial and ventricular arrhythmias Cardiac enlargement secondary to congenital heart defects Cardiomyopathy Neoplasia Trauma Systemic diseases Conduction system disease Cardiomyopathy Neoplasia Idiopathic fibrosis in older cats Dogs and Cats Acidosis or alkalosis Autonomic nervous system imbalance (parasympathetic or sympathetic); central nervous system (pain, excitement, fear); respiratory, gastrointestinal, organic brain disease Drug toxicity (e.g., digitalis, preoperative sedatives, anesthetic agents, catecholamines, antiarrhythmic agents, bronchodilators) Electrolyte disorders (hyperkalemia, hypercalcemia, hypokalemia, hypocalcemia, hypomagnesemia) Endocrinopathies (hypothyroidism, hyperthyroidism, Addison’s disease, pheochromocytoma) Hypothermia Hypovolemia Hypoxia, anemia Mechanical stimulation (cardiac catheterization, intravenous catheter) Neoplasia Shock Toxemia, sepsis Trauma MECHANISMS • The normal cardiac impulse is generated automatically in the sinus node and is spread through the atria rapidly and sequentially via the His bundle, the bundle branches, and the intraventricular conduction system to the ventricular myocardium. • The normal atrioventricular (AV) node serves as a bridge between the atria and the ventricles and slows the cardiac impulse prior to rapid impulse conduction through the ventricles. • Cardiac rhythm disturbances develop from diverse electrophysiologic mechanisms. • Enhanced automaticity in sinus node or subsidiary pacemaker cells can generate tachycardias or ectopic rhythms. Such activity may be influenced by sympathetic activity. • Triggered activities are common causes of ectopic rhythms and tachycardias. Early or late afterdepolarizations follow a previously driven (sinus) depolarization. The premature impulses occur when the cell spontaneously depolarizes during or just after repolarization. • Reentry, a common arrhythmia mechanism, typically is caused by functional dissociation of cardiac tissue, a unidirectional block in one pathway, and slowed conduction in the other pathway. The impulse then returns to the origination point by retrograde conduction through the unidirectionally blocked pathway. • Electrophysiologic mechanisms, arrhythmia manifestations and accompanying clinical signs and symptoms may vary widely among dogs with specific inherited cardiac diseases. Arrhythmogenic mechanisms can be modified (for better or for worse) by autonomic activity, heart rate, and many cardiac and non-cardiac drugs. DIAGNOSTIC APPROACH Systematic Evaluation of the Electrocardiographic Strip See also Chapter 144 . • Is sinus rhythm or an arrhythmia present? • Is the heart rate rapid, slow, or normal? • Are P waves present? • Yes. Do the P (atria) waves occur at regular or irregular intervals? What are the height, width, and direction? • No. What reason or abnormality explains the absence of the P wave? Is the P wave superimposed on a portion of the QRS complex, S-T segment, or T wave? Is the arrhythmia atrial standstill, atrial fibrillation, atrial flutter, AV junctional escape rhythm, or atrial tachycardia? • Do the QRS (ventricular) complexes occur with regularity and uniformity? What is their morphology? If wide and bizarre, is this due to a ventricular arrhythmia or caused by a premature atrial impulse that is aberrantly conducted, or is bundle branch block evident? • What is the relationship between the P waves and the QRS complexes? Is the relationship consistent? • If AV dissociation is present, from where does the QRS complex evolve? Are AV junctional and/or Purkinje or idioventricular foci involved? Questions To Be Answered in the Interpretation of Cardiac Arrhythmia • What is the possible mechanism for the arrhythmia? • Is it sinus, atrial, AV junctional, or ventricular in origin? • Is there a conduction abnormality? • What is the severity and frequency of the arrhythmia? SUPRAVENTRICULAR RHYTHMS Sinus Rhythm Definition • Impulses originate in the sinus node. • The rhythm is regular with less than a 10% variation in the R-R interval. • There is a normal P wave for each QRS complex, with a constant P-R interval. • The heart rate is between 60 and 180 beats per minute (bpm) in dogs and between 120 and 240 bpm in cats. Etiology and Clinical Significance • Sinus rhythm is a normal resting rhythm in dogs and cats and requires no therapy. • Animals with symptomatic cardiac disease or non-cardiac disease may show a sinus rhythm. Sinus Arrhythmia Definition • Impulses originate in the sinus node. • The rhythm is irregular with more than a 10% variation in the R-R interval. • There is a normal P wave for each QRS complex, with a constant P-R interval. • A wandering pacemaker (a change in the morphology of the P wave due to a change in pacemaker location or conduction) is often present. • Heart rates are similar to those for sinus rhythm. Etiology and Clinical Significance • Sinus arrhythmia is a normal rhythm variation in the resting dog, often correlated with varying levels of sinus node vagal tone, which changes with respiration (decreased vagal tone and increased heart rate during inspiration). • Sinus arrhythmia is unusual in cats. • Pronounced sinus arrhythmia occurs in the normal resting dog and in dogs and cats with respiratory disease. Treatment • No treatment is required unless there is symptomatic bradycardia, in which case anticholinergics or sympathomimetics may be helpful. Sinus Bradycardia Definition • Impulses originate in the sinus node but at a slower-than-normal frequency. • The rhythm is regular. • There is a normal P wave for each QRS complex, with a constant P-R interval. • The heart rate is <70 bpm in dogs (<60 bpm in giant breeds) and <120 bpm in cats. Etiology and Clinical Significance • Sinus bradycardia may be a normal physiologic rhythm variation resulting from high levels of resting vagal tone. • Hypothyroidism, sinus node disease (sick sinus syndrome), elevated cerebrospinal fluid pressure, and hypothermia are among the pathologic causes of sinus bradycardia. • Drugs that can cause a sinus bradycardia include acepromazine, xylazine, other alpha 2 -agonists (e.g., medetomidine), narcotics, digoxin, beta-blockers, diltiazem, pilocarpine, and general anesthetics. • Animals with sinus bradycardia are often asymptomatic. • Clinical signs of weakness, lethargy, and syncope may accompany sinus bradycardia. Treatment • Asymptomatic dogs or cats require no specific therapy. • When correlated with signs of weakness or syncope, an atropine response test should be performed (0.04 mg/kg IM) followed by an electrocardiogram (ECG) in 15 to 30 minutes. • If there is an increase in the cardiac rate following atropine administration, the animal may benefit from oral anticholinergic agents ( Table 145-3 ). • A poor clinical response to atropine suggests the need for a temporary or permanent cardiac pacemaker in symptomatic animals. • CHF may also develop, in which case consider diuretics and vasodilators as adjunctive treatment. Digitalis may exacerbate the sinus bradycardia in these cases. Hypotension may develop from vasodilator therapy. Sinus Block and/or Sinus Arrest ( Fig. 145-1 ) Definition • A primary disorder of the sinus node resulting in lack of generation of the cardiac impulse or its poor propagation across surrounding tissue. • It is not possible to distinguish between sinus block and sinus arrest in dogs because of the normal variation in the R-R interval (sinus arrhythmia). • The heart rate is variable and is often correlated with a bradycardia or slow sinus arrhythmia. • The rhythm is regularly irregular or irregular with pauses. • There is a normal P wave for each QRS complex with a pause equal to or greater than 2 times the normal R-R interval. • The P wave may vary in shape if a concurrent wandering pacemaker is present. Figure 145-1 Sinus block and/or arrest with a ventricular escape beat in a dog. (Lead II rhythm strip, paper speed 50 mm/sec; 1 cm = 1 mV.) Etiology and Clinical Significance • Sinus arrest may be consistent with an increase in vagal tone (e.g., ocular pressure, irritation of the vagus nerve, brachycephalic breeds, or respiratory disease). • Diseases of the atria (including fibrosis, cardiomyopathy, and neoplasia) and drug toxicity (e.g., digitalis, propranolol, quinidine, xylazine, and acepro-mazine) may result in sinus arrest. • Sinus arrest is one of the arrhythmias of the sick sinus syndrome. Electrocardiographic Differentials • Sinus block and/or sinus arrest can be confused with marked sinus arrhythmia or with sinus bradycardia and non-conducted atrial premature complexes (APCs). Treatment • Treat the same as sinus bradycardia. Sinus Tachycardia Definition • Impulses originate in the sinus node but at a faster-than-normal frequency. • The rhythm is regular. • There are normal P waves for each QRS complex. • The heart rate is >140 bpm in the giant breeds, >180 bpm in toy-breed dogs, and >240 bpm in cats. Etiology and Clinical Significance • Sinus tachycardia may be a normal physiologic rhythm resulting from high sympathetic tone occurring with exercise or excitement. • Sinus tachycardia may also occur with conditions such as stress, anxiety, pain, shock, fever, anemia, CHF, hyperthyroidism, and pheochromocytoma. • Drugs (e.g., atropine, sympathomimetic agents, theophylline, ketamine, and light anesthesia) and intoxicants (caffeine, chocolate, cocaine) also can cause sinus tachycardia. Electrocardiographic Differentials • Other supraventricular tachyarrhythmias confused with sinus tachycardia include paroxysmal (atrial or AV junctional) tachycardia, atrial flutter with 2:1 AV block, and ventricular tachycardia when sinus tachycardia is associated with wide QRS complexes. • A vagal maneuver (e.g., carotid sinus or ocular stimulation for 5 to 10 seconds) may result in a transient, gradual slowing of the sinus tachycardia. Treatment • Identify and treat the underlying cause of the sinus tachycardia. • Antiarrhythmic drugs are seldom required. • Administer atenolol (6.25 mg total dose q12-24h PO) or propranolol (2.5 mg total dose q8-12h PO) to hyperthyroid cats with intractable tachycardia (i.e., unresponsive to antithyroid medication). • Administer digitalis for sinus tachycardia in CHF. Digoxin will reestablish normal baroreceptor function and lessen sympathetic tone. Atrial Premature Complexes ( Fig. 145-2 ) Definition • Impulses originate from an atrial focus, often other than the sinus node. • The rhythm is irregular and the heart rate varies with the sinus node rate. • There is usually an abnormal P’ wave (premature P wave) followed by a normal QRS complex. The P’-R interval of the APC may vary from the sinus rhythm P-R interval. The P’ wave may have various morphologies and may be fused with the T wave of the preceding beat. • The P′ wave may occur so early in the cardiac cycle that the AV conduction system will be refractory and the impulse will not be conducted to the ventricles (e.g., APC with physiologic AV block). • The pause following the APC is often less than fully compensatory because of premature depolarization and resetting of the sinus node. Full compensatory pause occurs when the R wave-to-R wave interval surrounding the APC is equal to two normal R-R intervals. • The QRS complex is usually normal, but the intraventricular conduction system may be, in a relative or absolute refractory period, causing a bizarre (abnormal shape or direction) QRS complex. This abnormality is termed an APC with aberrant ventricular conduction. Figure 145-2 Atrial premature complexes in a dog. (Lead II rhythm strip, paper speed 50 mm/sec; 1 cm = 1 mV.) Etiology and Clinical Significance • APCs often indicate underlying cardiac disease (e.g., chronic valvular fibrosis, cardiomyopathy, congenital defect, or cor pulmonale) resulting in atrial enlargement. • Other causes include electrolyte disturbances, thyrotoxicosis, hypoxia, anemia, drug toxicity (e.g., digitalis, dobutamine, or dopamine), toxemia, and increased sympathetic tone. Electrocardiographic Differentials • Sinus rhythm with APCs may be confused with marked sinus arrhythmia and ventricular premature complexes during auscultation, and with ventricular premature complexes on the ECG, when APCs are conducted with aberrant ventricular conduction. • A P′ wave preceding the abnormal QRS complex and a similarity of the initial deflection of the QRS complex compared with a preceding normal beat supports the diagnosis of aberrant conduction. Treatment • Infrequent APCs may be a normal variation and do not require treatment. • If this arrhythmia is associated with CHF, treat the arrhythmia with digoxin. • If the APCs are associated with poor hemodynamic status without myocardial failure, prescribe digoxin, diltiazem, or a beta-blocker (e.g., propranolol or atenolol) (see Table 145-3 ). Atrial Tachycardia ( Figs. 145-3 and 145-4 ) Definition • Atrial tachycardia indicates rapid, abnormal impulses originating from an atrial site other than the sinus node. • The atrium and/or AV junctional areas may be involved in a reentrant circuit that allows the impulse to restimulate the atrium, as well as to pass to the ventricles. (A vagal maneuver may abolish this arrhythmia.) • An abnormal automatic focus in the atrium may also be responsible for this arrhythmia. (A vagal maneuver will cause AV block but not abolish the atrial tachycardia.) • The heart rate is >140 to 180 bpm in dogs and >240 bpm in cats, in which it often approaches 300 bpm. • The rhythm is usually regular but may be slightly irregular. • There is a P′ wave for each QRS complex, although the P′ wave is usually of different morphology than the sinus P wave. The P-R interval is constant. • The P′ wave may not be evident because it may be fused with the preceding T wave or occur simultaneously with the preceding QRS complex. • The QRS complex may also be of different morphology because of aberrant ventricular conduction. • An irregular R-R interval may be caused by concurrent AV block or by multifocal atrial tachycardia (P’ waves varying in shape, the firing of two or more ectopic atrial foci).
Share this:
- Click to share on Twitter (Opens in new window)
- Click to share on Facebook (Opens in new window)
Related posts:
- Feline Infectious Peritonitis (Feline Coronavirus)
- Borreliosis (Lyme Disease)
- Immune-Mediated Dermatoses
- Otitis Media and Otitis Interna
Stay updated, free articles. Join our Telegram channel
Comments are closed for this page.

Full access? Get Clinical Tree

Search Filters:
Phone Numbers
Routine and emergency care.
Companion Animal Hospital in Ithaca, NY for cats, dogs, exotics, and wildlife
Equine and Nemo Farm Animal Hospitals in Ithaca, NY for horses and farm animals
Cornell Ruffian Equine Specialists, on Long Island for every horse
Ambulatory and Production Medicine for service on farms within 30 miles of Ithaca, NY
Animal Health Diagnostic Center New York State Veterinary Diagnostic Laboratory
General Information
Cornell University College of Veterinary Medicine Ithaca, New York 14853-6401

In this section :
- "Leaky Valve Disease" of Older Dogs
- Aortic/Subaortic Stenosis
- Holter Monitoring
- Canine Dilated Cardiomyopathy (DCM)
- Companion Animal Hospital - Cardiology - Meet Our Specialists
- Hypertrophic Cardiomyopathy (HCM)
- Patent Ductus Arteriosus
- Pulmonic Stenosis in Dogs
- Companion Animal Hospital
Arrhythmias (Abnormal Rhythms) in Dogs
What dogs get arrhythmias .
All breeds and ages of dogs can get arrhythmias. Some specific arrhythmias are identified in specific breeds. The cause and the treatment vary widely depending on the diagnosis.
Ventricular Arrhythmias
Boxers, bulldogs, German shepherds
A common disease identified in boxers is arrhythmogenic right ventricular cardiomyopathy or ARVC. This is commonly called "Boxer Cardiomyopathy". The arrhythmia seen in these dogs is primarily from the right ventricle, but they may also come from other locations in the heart. Bulldogs also get a variation of this disorder. These ventricular arrhythmias may occur in rapid succession and this is called ventricular tachycardia. When ventricular tachycardia occurs it may lead to a decrease in blood flow to the body. When the perfusion to the brain decreases enough dogs may collapse. This arrhythmias may degenerate into ventricular fibrillation which is a fatal abnormal rhythm. Therefore, some dogs with ventricular arrhythmias must be treated with antiarrhythmics (e.g. sotalol). Most often to determine if this is required electrocardiograms are recorded and 24-hour electrocardiograms are monitored ( Holter monitoring ). These same tests are used to monitor the response to treatment. In addition to treating the arrhythmias associated with ARVC, dogs need to have other diagnostics to understand the extent of the structural and functional problems in addition to the electrical disorder of the arrhythmia.
German shepherds have an inherited ventricular arrhythmia that affects young dogs between 3 and 24 months of age. Some dogs die suddenly of these arrhythmias most commonly between 5 and 9 months of age. A diagnosis usually requires a Holter monitoring period to catch the dangerous arrhythmia. After 24 months of age the arrhythmias disappear and the dogs are no longer at risk, although when used for breeding they have affected offspring when mated to a dog that has the genetic background of risk.
Atrial Fibrillation
Great Danes, Doberman Pinschers, Newfoundlands, Irish Wolfhounds, Boxers and other large breeds
Sick Sinus Syndrome
West Highland White Terriers, Dachshunds, Miniature Schnauzers, Boxers, Cocker spaniels
Sick sinus syndrome is characterized by a heart rhythm whereby the sinus node (which normally initiates the beating heart) does not discharge an impulse to trigger the heart to contract. As a result the heart literally stops beating. If the heart stops for more than 8 seconds then the dog will collapse/faint. Sometimes the heart will have another part of the heart initiate a beat to rescue the heart from complete arrest. Most of the time the sinus node will eventually start up again to do its job but the dog has a rhythm with many long pauses. Some dogs with sick sinus syndrome have a more constant sinus bradycardia (too slow) because the sinus node has a low firing rate. Other dogs with sick sinus syndrome will have periods of excessive tachycardia (rapid rate) in addition to the pauses or bradycardia. When a dog has clinical signs of sick sinus syndrome it is almost always required that a pacemaker be implanted. The implantation of a pacemaker is today a common procedure in dogs. Veterinary cardiologists who are experienced in the implantation of pacemakers and the programming of these pacemakers can best insure the best treatment for afflicted dogs. The response to treatment is usually very good. In dogs that also have the tachycardia this is treated with medication(s) if it does not subside after pacing.
Heart Block
Any breed, may affect cats too
Myocarditis
Any breed but often medium to larger dogs
- Some dogs with ventricular arrhythmias or heart block may have underlying inflammation of the heart known as myocarditis. In these situations monitoring of the arrhythmia and other tests (e.g. echocardiography, troponin I, C-reactive protein) are required. Often the cause of the myocarditis cannot be identified, but this disease quite often must be handled carefully because often dogs die suddenly when affected. Treatment includes antiarrhythmic medications and anti-inflammatory drugs such as corticosteroids.
The Dog Electrocardiogram: A Critical Review
- Reference work entry
- pp 1861–1908
- Cite this reference work entry

- David K. Detweiler 7
3894 Accesses
2 Citations
1 41.1 History and Literature
Electrocardiographic studies in dogs date back to the pioneering investigations of Augustus Waller [ 1 ] with the capillary electrometer and Willem Einthoven’s development of the string galvanometer electrocardiograph [ 2 – 4 ]. As late as 1914 [ 5 ], Waller considered electrocardiography (ECG) as an experimental method, useful to physiologists rather than as a clinical tool for physicians. However, clinical application in man had already started and advanced rapidly (Lewis, 1909–1925 [ 6 ]; Rothberger, 1912–1930 [ 7 ]; Winterberg, 1912–1930 [ 9 ]; Scherf, 1921–present [ 8 ]; Wenckebach, 1899–1930 [ 9 ]; and Wilson, 1919–1945 [ 10 ]). Clinical use in canine medicine was modest in those early days (Nörr, 1913–1931 [ 11 ]; Roos, 1925 [ 12 ]; Haupt, 1929 [ 13 ]; Ludwig, 1924 [ 14 ]; and Gyarmati, 1939 [ 15 ]) and subsequently, until Nils Lannek’s systematic study and statistical analysis of clinical records from healthy and diseased dogs [ 16 ]. Lannek also introduced a precordial-lead...
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Waller, A., Introductory address on the electromotive properties of the human heart. Br. Med. J ., 1888; 2 : 751–754.
PubMed CAS Google Scholar
Einthoven, W., Enregistrement galvanométrique de l’électrocardiogramme humain et contrôle des résultats obtenus par l’emploi de l’électromètre capillaire en physiologie. Arch. Néerland. Sci. Not ., 1904; 11 (9): 202–209.
Google Scholar
Einthoven, W., Weiteres über das Elektrokardiogramm. Pfluegers Arch ., 1908; 122 : 517–584.
Einthoven, W, G. Fahr, and A. de Waart, Über die Richtung und die manifeste Grösse der Potentialschwankungen immenschlichen Herzen und über den Einfluss der Herzlage auf die Form des Elektrokardiogramms. Pfluegers Arch ., 1913; 150 : 275–315.
Waller, A.D., A Short Account of the Origin and Scope of Electrocardiography. The Harvey Lectures, 1913/14 . Philadelphia, PA: Lippincott, 1915, pp. 17–33.
Lewis, T., The Mechanism and Graphic Registration of the Heart Beat , 3rd edn. London: Shaw, 1925.
Rothberger, C.J., Normale und pathologische Physiologie der Rhythmik unct Koordination des Herzens. Erg. Physiol ., 1931; 32 : 472–820.
Scherf, D. and A. Schott, Extrasysto/es and Allied Arrhythmias , 2nd edn. Chicago, IL: Year Book Medical, 1973.
Wenckebach, K.F. and H. Winterberg, Die unregelmässigeHerztätigket . Leipzig: Engelman, 1927.
Wilson, F.N., F.D. Johnston, and E. Lepeschkin, Editors. Selected Papers . Ann Arbor, MI: Heart Station, University Hospital, 1954.
Nörr, J., Über Herzstromkurvenaufnahmen an Haustieren. Zur Einführung der Elektrokardiographie in die Veterinärmedizin. Arch. Wiss. Prakt. Tierheilkd ., 1922; 48 : 85–111.
Roos, J., Vorhofflimmern bei den Haustieren. Arch . Wiss. Prakt. Tierheilkd ., 1924; 51 : 280–293.
Haupt, K., Die Aufnahmetechnik des Hundeelektrokardiogramms in der Veterinärklinik und ihre Ergebnisse , dissertation. Giessen: University of Giessen, 1929.
Ludwig, K-H., Die Elektrokardiographie beim gesunden Hund unter besonderer Berücksichtigung ihrer Anwendung in der Klinik , dissertation. Leipzig: University of Leipzig, 1924.
Gyarmati, E., Klinische elektrokardiographische Untersuchungen beim Hunde , dissertation. Budapest: University of Budapest, 1939.
Lannek, N.A., Clinical and Experimental Study on the Electrocardiogram in Dogs , dissertation. Stockholm: Royal Veterinary College, 1949.
Burch, G.E. and N.P. DePasquale, A History of Electrocardiography . Chicago, IL: Year Book Medical, 1964.
Bellet, S., Clinical Disorders of the Heart Beat , 2nd edn. Philadelphia, PA: Lea & Febiger, 1963.
Bellet, S., Essentials of Cardiac Arrhythmias: Diagnosis and Management . Philadelphia, PA: Saunders, 1972.
Schamroth, L., The Disorders of Cardiac Rhythm , vols. 1, 2, 2nd edn. London: Blackwell Scientific, 1980.
Ettinger, S.J. and P.F. Suter, Canine Cardiology . Philadelphia, PA: Saunders, 1970.
Detweiler, D.K., D.F. Patterson, J.W. Buchanan, and D.N. Knight, The cardiovascular system, in Canine Medicine, vol. 2, 4th edn., E.J. Catcott, Editor. Santa Barbara, CA: American Veterinary, 1979, pp. 813–949.
Bolton, G.R., Handbook of Canine Electrocardiography . Philadelphia, PA: Saunders, 1975.
Tilley, L.P., Essentials of Canine and Feline Electrocardiography , 2nd edn. Philadelphia, PA: Lea & Febiger, 1985.
Detweiler, D.K., The use of electrocardiography in toxicological studies with Beagle dogs, in Cardiac Toxicology , vol. 3, T. Balazs, Editor. Boca Raton, FL: CRC Press, 1981, pp. 33–82.
Lautenschlager, O., Grundlagen der Aufnahmetechnik des Elektrokardiogrammes von Pferd und Rind und ihre Ergebnisse , dissertation. Giessen: University of Giessen, 1928.
Nehb, W., Zur Standardisierung der Brustwandableitungen des Elektrokardiogramms. Klin. Wchnschr ., 1938; 17 : 1807–1811. Cited by Lepeschkin, E., Modern Electrocardiography , vol. 1. Baltimore, MD: Williams & Wilkins, 1951.
Spörri, H., Der Einfluss der Tuberkulose auf das Elektrokardiogramm. (Untersuchungen an Meerschweinchen und Rindern.) Arch. Wiss. Prakt. Tierheilkd ., 1944; 79 : 1–57.
Detweiler, D.K., The use of electrocardiography in toxicological studies with rats, in The Rat Electrocardiogram in Pharmacology and Toxicology , R. Budden, D.K. Detweiler, and G. Zbinden, Editors. Oxford: Pergamon, 1981, pp. 83–115.
Hellerstein, H.K. and R. Hamlin, QRS component of the spatial vectorcardiogram and of the spatial magnitude and velocity electrocardiograms of the normal dog. Am. J. Cardiol ., 1960; 6 : 1049–1061.
Hahn, A.W., R.L. Hamlin, and D.F. Patterson, Standards for canine electrocardiography. Academy of Veterinary Cardiology Committee Report , 1977.
Kraus, M.S., N.S. Moise, M. Rishniw, et al. Morphology of ventricular arrhythmias in the boxer as measured by 12-lead electrocardiography with pace-mapping comparison. J. Vet. Intern. Med ., 2002; 16 (2): 153–158.
PubMed Google Scholar
McFee, R. and A. Parungao, An orthogonal lead system for clinical electrocardiography. Am. Heart J ., 1961; 62 : 93–100.
Bloch, W.N. Jr., K.A. Busch, and T.R. Lewis, The Frank vectorcardiogram of the Beagle dog. J. Electrocardiol ., 1972; 5 : 119–125.
Bojrab, M.J., J.E. Breazile, and R.D. Morrison, Vectorcardiography in normal dogs using; the Frank lead system. Am. J. Vet. Res ., 1971; 32 : 925–934.
Morita, H., Electrocardiograms of conscious Beagle dogs by apex-base bipolar lead. Adv. Anim. Cardiol ., 1984; 17 : 19–23.
Sugano, S., Electrocardiographic studies in the beagle as an experimental dog. Adv. Anim. Electrocardiography , 1977; 10 :45–50.
Takahashi, M., Experimental studies on the electrocardiogram of the dog. Jpn. J. Vet. Sci ., 1964; 24 : 191–210.
Cagan, S., Prespevok k elektrokardiogramu, psa. Bratisl. Lek. Listy ., 1959; 39 : 540–545.
Cagan, S. and E. Barta, Die Bedingungen des konstanten Elektrokardiogrammes beim Hund. Z. Kreislaufforsch ., 1959; 48 : 1101–1105.
Hulin, I. and S. Rippa, Why does the electrocardiogram of the dog change with a change in the foreleg position? Am. Heart J ., 1970; 79 : 143.
Hill, J.D., The significance of foreleg positions in the interpretation of electrocardiograms and vectorcardiograms from research animals. Am. Heart J ., 1968; 75 (4): 518–527.
Hill, J.D., The electrocardiogram in dogs with standardized body and limb positions. J. Electrocardiol ., 1968; 1 : 175–182.
Almasi, J.J., O.H. Schmitt, and E.F. Jankus,. Electrical characteristics of commonly used canine ECG electrodes. Proc. Annu. Conf. Eng. Med. Biol ., 1970; 12 : 190.
Rydén, L., A. Waldenström, and S. Holmberg, The reliability of intermittent ECG sampling in arrhythmia detection. Circulation , 1975; 52 : 540–545.
Morganroth, J., Ambulatory monitoring: the impact of spontaneous variability of simple and complex ventricular ectopy, in Cardiac Arrhythmias , D.G. Harrison, Editor. Boston, MA: Hall, 1981, pp. 479–492.
Moïse, N.S., Diagnosis and management of canine arrhythmias, in Canine and Feline Cardiology , 2nd edn., P.R. Fox, D.D. Sisson, N.S. Moïse, Editors. Philadelphia, PA: W. B. Saunders, 1999, pp. 331–385.
Grauwiler, J., Herz und Kreislauf der Säugetiere: Vergleichend-Funktionelle Daten . Batiel: Birkhäuser,1965.
Bazett, H.C., An analysis of the time relations of electrocardiograms. Heart , 1920; 7 : 353–370.
Chiang, A.Y., D.L. Holdsworth, and D.J. Leishman, A one-step approach to the analysis of the QT interval in conscious telemetrized dogs. J. Pharmacol. Toxicol. Methods , 2006 Mar 6; E-print.
Miyazaki, H., H. Watanabe, T. Kitayama, M. Nishida, Y. Nishi, K. Sekiya, H. Suganami, and K. Yamamoto, QT PRODACT: sensitivity and specificity of the canine telemetry assay for detecting drug-induced QT interval prolongation. J. Pharmacol. Sci ., 2005; 99 (5): 523–529.
Gauvin, D.V., L.P. Tilley, F.W. Smith Jr,, and T.J. Baird, Electrocardiogram, hemodynamics, and core body temperatures of the normal freely moving laboratory beagle dog by remote radiotelemetry. J. Pharmacol. Toxicol. Methods , 2006; 53 (2): 128–139.
Watanabe, H. and H. Miyazaki, A new approach to correct the QT interval for changes in heart rate using a nonparametric regression model in beagle dogs. J. Pharmacol. Toxicol. Methods , 2006; 53 (3): 234–241.
Camm, A.J., Clinical trial design to evaluate the effects of drugs on cardiac repolarization: current state of the art. Heart Rhythm , 2005; 2 (2 Suppl): S23–29. Review.
Harada, T., J. Abe, M. Shiotani, Y. Hamada, and I. Horii, Effect of autonomic nervous function on QT interval in dogs. J. Toxicol. Sci ., 2005; 30 (3): 229–237.
Batchivarov, V.N. and M. Makik, There is little sense in “common” QT correction methods. J. Cardiovasc. Electrophysiol ., 2005; 16 (7): 809.
Tattersall, M.L., M. Dymond, T. Hammond, and J.P. Valentin, Correction of QT values to allow for increases in heart rate in conscious Beagle dogs in toxicology assessment. J. Pharmacol. Toxicol. Methods ., 2006; 53 : 11–19.
Lewis, T., J. Meakins, P.D. White, The excitatory process in the dog’s heart. Part I. The auricles. Philos. Trans. R. Soc. Lond. Ser. B , 1914; 205 : 375–420.
Meek, W.J. and J.A.E. Eyster, Experiments on the origin and propagation of the impulse in the heart. IV. The effect of vagal stimulation and of cooling on the location of the pacemaker within the sino-auricular node. Am. J. Physiol ., 1914; 34 : 368–383.
Hinds, M.H., D.R. Clark, J.D. McCrady, and L.A. Geddes, The relationship among pacemaker location, heart rate, and P-wave configuration in the dog. J. Electrocardiol ., 1972; 5 : 56–64.
Goldberg, J.M. and M.H. Lynn-Johnson, Changes in canine P wave morphology observed with shifts in intra-SA nodal pacemaker localization. J. Electrocardiol ., 1980; 13 : 209–217.
Boineau, J.P., R.B. Schuessler, and C.R. Mooney, et al., Multicentric origin of the atrial depolarization wave: the pacemaker complex. Circulation , 1978; 58 : 1036–1048.
Anonymous (editorial). U waves: unimportant undulations? Lancet , 1983; 2 : 776–777.
Watanabe, Y. and H. Toda, The U wave and aberrant intraventricular conduction. Further evidence for the Purkinje repolarization theory on genesis of the U wave. Am. J. Cardiol ., 1978; 41 : 23–31.
Kishida, H., J.S. Cole, and B. Surawicz, Negative U wave: A highly specific but poorly understood sign of heart disease. Am. J. Cardiol ., 1982; 49 : 2030–2036.
Rudolph, A.M., P.A.M. Auld, R.J. Golinko, and M.H. Paul, Pulmonary vascular adjustments in the neonatal period. Pediatrics , 1961; 28 : 28–34.
Averill, K.H., W.W. Wagner Jr., and J.H.K. Vogel, Correlation of right ventricular pressure with right ventricular weight. Am. Heart J ., 1963; 66 : 632–635.
Kirk, G.R., D.M. Smith, D.P. Hutcheson, and R. Kirby, Postnatal growth of the dog heart. J. Anat ., 1975; 119 : 461–469.
Trautvetter, E., Untersuchungen zur EKG-Entwicklung an gesunden Welpen und Welpen mitangeborenen Pulmonalstenosen, Habilitationsschrift . Berlin: Freie Universität Berlin, 1972.
Trautvetter, E., D.K. Detweiler, and D.F. Patterson, Evolution of the electrocardiogram in young dogs during the first 12 weeks of life. J. Electrocardiol ., 1981; 14 : 267–273.
Trautvetter, E., D.K. Detweiler, F.K. Bohn, and D.F. Patterson, Evolution of the electrocardiogram in young dogs with congenital heart disease leading to right ventricular hypertrophy. J. Electrocardiol ., 1981; 14 : 275–282.
Detweiler, D.K., The cardiovascular system, in Duke’s Physiology of Domestic Animals , chaps. 5 – 12 , 10th edn., M.J. Swenson, Editor Ithaca, NY: Cornell University Press, 1984, pp. 68–225.
Amend, J.F. and H.E. Hoff, Analysis of patterns and parameters of the respiratory heart rate response in the unanesthetized dog. Southwest. Vet ., 1970; 22 : 301–311.
Fuller, J.L., Genetic variability in some physiological constants of dogs. Am. J. Physiol ., 1951; 166 : 20–24.
Kleiger, R.E., P.K. Stein, and J.T. Bigger Jr., Heart rate variability: measurement and clinical utility. Ann. Noninvasive Electrocardiol ., 2005; 10 (1): 88–101. Review.
Werner, J., A. von Recum, E. Trautvetter, and H. Sklaschus, Über den Ruherhythmus des Herzens beim Hund. Z. Kreislaufforsch ., 1969; 58 : 593–600.
Lange, H., Ober den Eintritt der Atmungsarrhythmie in der ersten Lebenszeit des Hundes , dissertation. Munich: University of Munich, 1937.
Horan, L., G.E. Burch, and J.A. Cronvich, Spatial vectorcardiograms in normal dogs. Circ. Res ., 1957; 5 : 133–136.
Horan, L.G., G.E. Burch, and J.A. Cronvich, A study of the influence upon the spatial vectorcardiogram of localized destruction of the myocardium of dog. Am. Heart J ., 1957; 53 : 74–90.
Horan, L.G., G.E. Burch, and J.A. Cronvich, Spatial vectorcardiogram in dogs with chronic localized myocardial lesions. J. Appl. Physiol ., 1960; 15 : 624–628.
Hamlin, R.L., F.S. Pipers, and C.R. Smith, Computer methods for analysis of dipolar characteristics of the electrocardiogram. Am. J. Vet. Res ., 1968; 29 : 1867–1881.
Boineau, J.P., J.D. Hill, M.S. Spach, and E.N. Moore, Basis of the electrocardiogram in right ventricular hypertrophy: relationship between ventricular depolarization and body surface potentials in dogs with spontaneous RVH-contrasted with normal dogs. Am. Heart J ., 1968; 76 : 605–627.
Chastain, C.B., D.H. Riedesel, and P.T. Pearson, McFee and Parungao. Orthogonal lead vectorcardiography in normal dogs. Am. J. Vet. Res ., 1974; 35 : 275–280.
Bruninx, P. and H.E. Kulbertus, The McFee-Parungao vectorcardiogram in normal dogs. J. Electrocardiol ., 1974; 7 : 227–236.
Detweiler, D.K. and D.F. Patterson, The prevalence and types of cardiovascular disease in dogs. Ann. N. Y. Acad. Sci ., 1965; 127 : 481–516.
Hill, J.D., Electrocardiographic diagnosis of right ventricular enlargement in dogs. J. Electrocardiol ., 1971; 4 : 347–357.
Clark, D.R., J.G. Anderson, and C. Paterson, Imperforate cardiac septal defect in a dog. J. Am. Vet. Med. Assoc ., 1970; 156 : 1020–1025.
Bolton, G.R., S. Ettinger, and J.C. Roush II, Congenital peritoneopericardial diaphragmatic hernia in a dog. J. Am. Vet. Med. Assoc ., 1969; 155 : 723–730.
Patterson, D.F., Animal models of congenital heart disease (with special reference to patent ductus arteriosus in the dog), in Animal Models for Biomedical Research . Washington, DC: National Academy of Sciences Publication 1594, 1968, pp. 131–156.
Boineau, J.P., M.S. Spach, and J.S. Harris, Study of premature systoles of the canine heart by means of the spatial vectorcardiogram. Am. Heart J ., 1960; 60 : 924–935.
Bolton, G.R. and S.J. Ettinger, Right bundle branch block in the dog. J. Am. Vet. Med. Assoc ., 1972; 160 : 1104–1119.
Blake, D.F. and P. Kezdi, Vectorcardiography in uncomplicated canine bundle branch block. Circulation , 1961; 24 : 888–889.
De Micheli, A., G.A. Medrano, and D. Sodi-Pallares, Etude électro-vectocardiographique des blocs de branche chez le chien àla lumière du processus d’activation ventriculaire. Acta Cardiol ., 1963; 18 : 483–514.
Moore, E.N., J.P. Boineau, and D.F. Patterson, Incomplete right bundle-branch block. An electrocardiographic enigma and possible misnomer. Circulation , 1971; 44 : 678–687.
Littlewort, M.C.G., Canine electrocardiography; some potentialities and limitations of thetechnique. J. Small Anim. Pract ., 1967; 8 : 437–458.
Pyle, R.L., A Study of Certain Clinical, Genetical, and Pathological Aspects of Congenital Fibrous Subaortic Stenosis in the Dog , thesis. Philadelphia, PA: University of Pennsylvania, 1971.
Tilley, L.P., Essentials of Canine and Feline Electrocardiography: Interpretation and Treatment , 2nd edn. Philadelphia, PA: Lea & Febiger, 1985.
Rosenbaum, M.B., M.V. Elizari, and J.O. Lazzari, The Hemiblocks . Oldsmar, FL: Tampa Tracings, 1970.
Okuma, K., ECG and VCG changes in experimental hemiblock and bifascicular block. Am. Heart J ., 1976; 92 : 473–480.
Glomset, D.J. and A.T.A. Glomset, A morphologic study of the cardiac conduction system in ungulates, dog, and man. I and II. Am. Heart J ., 1940; 20 : 389–98, 677–701.
Patterson, D.F., D.K. Detweiler, K. Hubben, and R.P. Botts, Spontaneous abnormal cardiac arrhythmias and conduction disturbances in the dog. A clinical and pathologic study of 3,000 dogs. Am. J. Vet Res ., 1961; 22 : 355–369.
Long, D.M., R.C. Truex, K.R. Friedmann, A.K. Olsen, and S.J. Phillips, Heart rate of the dog following autonomic denervation. Anat. Rec ., 1958; 130 : 73–89.
Hoffman, B.F. and P.F. Cranefield, The physiological basis of cardiac arrhythmias. Am. J. Med ., 1964; 37 : 670–684.
Tse, W.W., Evidence of presence of automatic fibers in the canine atrioventricular node. Am. J. Physiol ., 1973; 225 : 716–723.
Damato, A.N. S.H. Lau, His bundle rhythm. Circulation , 1969; 40 : 527–534.
Jalife, J. and G.K. Moe, Effect of electrotonic potentials on pacemaker activity of canine Purkinje fibers in relation to parasystole. Circ. Res ., 1976; 39 : 801–818.
Jalife, J. and G.K. Moe, A biologic model of parasystole. Am. J. Cardiol ., 1979; 43 : 761–712.
Moe, G.K., J. Jalife, W.J. Mueller, and B. Moe, A mathematical model of parasystole and its application to clinical arrhythmias. Circulation , 1977; 56 : 968–979.
Furuse, A., G. Shindo, H. Makuuchi, et al. Apparent suppression of ventricular parasystole by cardiac pacing. Jpn. Heart J ., 1979; 20 : 843–851.
Castellanos, A., E. Melgarejo, R. Dubois, and R.M. Luceri, Modulation of ventricular parasystole by extraneous depolarizations. J. Electrocardiol ., 1984; 17 : 195–198.
Soloff, L.A., Parasystole, in Cardiac Arrhythmias , L.S. Dreifus and W. Likoff, Editors. New York: Grune & Stratton, 1973, pp. 409–415.
Meurs, K.M., Boxer dog cardiomyopathy: an update. Vet . Clin. North Am. Small Anim. Pract ., 2004; 34 (5): 1235–1244.
Baumwart, R.D., K.M. Meurs, C.E. Atkins, et al., Clinical, echocardiographic, and electrocardiographic abnormalities in Boxers with cardiomyopathy and left ventricular systolic dysfunction: 48 cases (1985–2003). J. Am. Vet. Med. Assoc ., 2005; 226 (7): 1102–1104.
Basso, C., P.R. Fox, K.M. Meurs, et al., Arrhythmogenic right ventricular cardiomyopathy causing sudden cardiac death in boxer dogs: a new animal model of human disease. Circulation , 2004; 109 (9): 1180–1185.
Kraus, M.S., N.S. Moise, M. Rishniw, et al., Morphology of ventricular arrhythmias in the boxer as measured by 12-lead electrocardiography with pace-mapping comparison. J. Vet. Intern. Med ., 2002; 16 (2): 153–158.
Meurs, K.M., A.W. Spier, N.A. Wright, et al., Comparison of the effects of four antiarrhythmic treatments for familial ventricular arrhythmias in Boxers. J. Am. Vet. Med. Assoc ., 2002; 221 (4): 522–527.
Moise, N.S., From cell to cageside: autonomic influences on cardiac rhythms in the dog. J. Small Anim. Pract ., 1998; 39 (10): 460–468.
Harpster, N.K., Boxer cardiomyopathy. A review of the long-term benefits of antiarrhythmic therapy. Vet. Clin. North Am. Small Anim. Pract ., 1991; 21 (5): 989–1004.
Moïse, N.S., V. Meyers-Wallen, W.J. Flahive, et al., Inherited ventricular arrhythmias and sudden death in German shepherd dogs. J. Am. Coll. Cardiol ., 1994; 24 : 233–243.
Moïse, N.S., P.F. Moon, W.J. Flahive, et al., Phenylephrine induced ventricular arrhythmias in dogs with inherited sudden death. J. Cardiovasc. Electrophysiol ., 1996; 7 : 217–230.
Gilmour, R.F. Jr. and N.S. Moïse, Triggered activity as a mechanism for inherited ventricular arrhythmias in German shepherd dogs. J. Am. Coll. Cardiol ., 1996; 27 : 1526–1533.
Moïse, N.S. and R.F. Gilmour Jr., and M.L. Riccio, An animal model of sudden arrhythmic death. J . Cardiovasc. Electrophysiol ., 1997; 8 : 98–103.
Moïse, N.S., D.A. Dugger, D. Brittain, et al., Relationship of ventricular tachycardia to sleep/wakefulness in a model of sudden cardiac death. Ped. Res ., 1996; 40 : 344–350.
Moïse, N.S., R.F. Gilmour Jr., M.L. Riccio, et al., Diagnosis of inherited ventricular tachycardia in German shepherd dogs. Am. J. Vet. Med. Assoc ., 1997; 210 : 403–410.
Freeman, L.C., L.M. Pacioretty, and N.S. Moïse, et al., Decreased density of I to in left ventricular myocytes from German shepherd dogs with inherited arrhythmias. J. Cardiovasc. Electrophysiol ., 1997; 8 : 872–883.
Dae, M., P. Ursell, R. Lee, C. Stilson, M. Chin, and N.S. Moïse, Heterogeneous sympathetic innervation in German shepherd dogs with inherited ventricular arrhythmias and sudden death. Circulation , 1997; 96 : 1337–1342.
Moïse, N.S., M.J. Riccio, W.J. Flahive, et al., Age dependent development of ventricular arrhythmias in a spontaneous animal model of sudden cardiac death. Cardiovasc. Res ., 1997; 34 : 483–492.
Riccio, M.L., N.S. Moïse, N.F. Otani, et al., Vector quantization of T wave abnormalities associated with a predisposition to ventricular arrhythmias and sudden death. Ann. Noninvasive Electrocardiol ., Jan 1998; 3 (1): 46–53.
Moïse, N.S., From cell to cageside cardiac rhythms in the dog: autonomic influence. J. Small Anim. Pract ., 1998; 39 : 460–468.
Sosunov, E.A., E.P. Anyukhovsky, A. Shvilkin, M. Hara, S.F. Steinberg, P. Danilo Jr., M.R. Rosen, N.S. Moïse, et al., Abnormal cardiac repolarization and impulse initialization in German shepherd dogs with inherited ventricular analysis and sudden death. Cardiovasc. Res ., 1999; 42 : 65–79.
Moïse, N.S., Inherited arrhythmias in the dog: potential experimental models of cardiac disease. Cardiovasc. Res ., 1999; 44 : 37–46.
Merot, J., V. Probst, M. Debailleul, U. Gerlacin, N.S. Moïse, et al., Electropharmacological characterization of cardiac repolarization in German shepherd dogs with an inherited syndrome of sudden death. J. Am. Coll. Cardiol ., 2000; 36 : 939–947.
Sosunov, E.A., R.Z. Gainullin, N.S. Moïse, et al., β 1 and β 2 –Adrenergic receptor subtype effects in German shepherd dogs with inherited lethal ventricular arrhythmias. Cardiovasc. Res ., 2000; 48 : 211–219.
Steinberg, S.F., S.A. Alcott, E. Pak, D.H. Hu, L. Protas, N.S. Moïse, et al., Beta-receptors increase in cAMP and include abnormal CAI cycling in the German shepherd sudden death model. Am. J. Physiol. Heart Circ. Physiol }., 2002; 282 : H1181–H1188.
Obreztchikova, M.N., E.A. Sosunov, E.P. Anyukhovsky, N.S. Moïse, et al., Heterogeneous ventricular repolarization provides a substrate for arrhythmias in German shepherd model of spontaneous arrhythmic death. Circulation , 2003; 108 : 1389–1394.
Sosunov, E.A., M.N. Obreztchikova, E.P. Anyukhovsky, N.S. Moïse, et al., Mechanisms of alph adrenergic potentiation of ventricular arrhythmias in German shepherd dogs with inherited arrhythmic sudden death. Cardiovasc. Res ., 2004; 61 : 715–723.
Protas, L., E.A. Sosunov, E.P. Anyukhovsky, N.S. Moïse, et al., Regional dispersion of L-type calcium current in ventricular myocytes of German shepherd dogs with lethal cardiac arrhythmias. Heart Rhythm , 2005; 2 : 172–176.
Gelzer, A.R.M., N.S. Mose, and M.L. Koller, Defibrillation of German shepherds with inherited ventricular arrhythmias and sudden death. J. Vet. Cardiol ., 2005; 7 (2): 97–107.
Wald, R.W., M.B. Waxman, and J.M. Colman, Torsade de pointes ventricular tachycardia: a complication of disopyramide shared with quinidine. J. Electrocardiol ., 1981; 14 : 301–307.
Bardy, G.H., R.M. Ungerleider, W.M. Smith, and R.E. Ideker, A mechanism of Torsades de Pointes as observed in a dog model. Circulation , 1981; 64 (Suppl. 4): 218.
Boucher, M., C. Dubray, and P. Duchene-Marullaz, Long-term observation of atri, fl and ventricular rates in the unanesthetized dog with complete atrioventricular block. Pfluegers Arch ., 1982; 395 : 341–343.
CAS Google Scholar
Hurwitz, R.A., Effect of glucagon on dogs with acute and chronic heart block. Am. Heart J ., 1971; 81 : 644–649.
Reynolds, R.D. and J. Di Salvo, Effects of dl-propranolol on atrial and ventricular rates in unaesthetized atrioventricular blocked dogs. J. Pharmacol. Exp. Ther ., 1978; 205 : 374–381.
Robinson, J.L., W.C. Farr, and G. Grupp, Atrial rate response to ventricular pacing in the unanesthetized A-V blocked dog. Am. J. Physiol ., 1973; 224 : 40–45.
Averill, K.H. and L.E. Lamb, Electrocardiographic findings in 67,375 asymptomatic subjects. I. Incidence of abnormalities. Am. J. Cardiol ., 1960; 6 : 76–83.
Böhle, E., Blutgefässe, in Erkrankungen durch Arzneimittel , R. Heinz, Editor. Stuttgart: Thieme, 1966, pp. 170–187.
Wenzel, D.G., Drug induced cardiomyopathies. J. Pharm. Sci ., 1967; 56 : 1209–1224.
Selye, H., Experimental Cardiovascular Diseases , vols. 1, 2. Berlin: Springer, 1970.
Chung, E.K. and H.M. Dean, Diseases of the heart and vascular system due to drugs, in Drug-Induced Diseases , vol. 4, L. Meyler and H.M. Peck, Editors. Amsterdam: Excerpta Medica, 1972, pp. 345–381.
Davies, D.M. and R.G. Gold, Cardiac disorders, in Textbook of Adverse Drug Reactions , D.M. Davies, Editor. Oxford: Oxford University Press, 1977, pp. 81–102.
Bristow, M.R., Editor, Drug-Induced Heart Disease . Amsterdam: Elsevier, 1980.
Balazs, T., Editor, Cardiac Toxicology , vols. 1, 2, 3. Boca Raton, FL: CRC Press, 1981.
Van Stee, E.W., Editor, Cardiovascular Toxicology . New York: Raven, 1982.
Spitzer, J. J., Editor, Myocardial injury. Adv. Exp. Med. Biol; Ser ., New York: Plenum, 1983; 161 : 421–443.
Surawicz, B. and K.C. Lasseter, Effect of drugs on the electrocardiogram. Prog. Cardiovasc. Dis ., 1970; 13 : 26–55.
Surawicz, B., Relationship between electrocardiogram and electrolytes. Am. Heart J ., 1967; 73 : 814–834.
Surawicz, B., The pathogenesis and clinical significance of primary T-wave abnormalities, in Advances in Electrocardiography , R.C. Schlant and J. Hurst, Editors. New York: Grune & Stratton, 1972, pp. 377–421.
Vaughan Williams, E.M., Classification of anti-arrhythmic drugs, in Symposium of Cardiac Arrhythmias , E. Sandøe, E. Flensted-Jensen, and K.H. Olesen, Editors. Södertälje, Sweden: Astra, 1970, pp. 449–468.
Singh, B.N., J.T. Collett, and C.Y. Chew, New perspectives in the pharmacologic therapy of cardiac arrhythmias. Prog. Cardiovasc. Dis ., 1980; 22 : 243–301.
Harrison, D.G., Editor, Cardiac Arrhythmias: A Decade of Progress . Boston, MA: Hall, 1981.
Lazdunski, M. and J.F. Renaud, The action of cardiotoxins on cardiac plasma membranes. Annu. Rev. Physiol ., 1982; 44 : 463–473.
Detweiler, D.K., Electrocardiographic monitoring in toxicological studies: principles and interpretations, in Myocardial Injury , J.J. Spitzer, Editor, Adv . Exp. Med. Biol ., 1983; 161 : 579–607.
Reynolds, E.W. and C.R. Vander Ark, Quinidine syncope and the delayed repolarization syndromes. Mod. Concepts Cardiovasc . Dis ., 1976; 45 : 117–122.
Detweiler, D.K., Reversal of T Waves in Leads rV 2 and V 10 in Toxicity Trials Indicates Left Ventricular Subendocardial and Papillary Muscle Ischemia or Damage , Personal Observation. Philadelphia, PA: University of Pennsylvania, 1985.
Osborne, B.E. and B.D.H. Leach, The Beagle Electrocardiogram. Food Cosmet. Toxicol ., 1971; 9 : 857–864.
Download references
Acknowledgement
Dr. Sydney Moise at Cornell University contributed updates to this chapter.
Author information
Authors and affiliations.
University of Pennsylvania, Philadelphia, PA, USA
David K. Detweiler
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
University of Glasgow, Glasgow, UK
Peter W. Macfarlane
Radboud University Nijmegen, Nijmegen, The Netherlands
A. van Oosterom
Lund University, Lund, Sweden
Weill Cornell Medical College, New York, NY, USA
Paul Kligfield
University of Amsterdam, Amsterdam, The Netherlands
Michiel Janse
St. George's, University of London, London, UK
Rights and permissions
Reprints and permissions
Copyright information
© 2010 Springer-Verlag London Ltd.
About this entry
Cite this entry.
Detweiler, D.K. (2010). The Dog Electrocardiogram: A Critical Review. In: Macfarlane, P.W., van Oosterom, A., Pahlm, O., Kligfield, P., Janse, M., Camm, J. (eds) Comprehensive Electrocardiology. Springer, London. https://doi.org/10.1007/978-1-84882-046-3_41
Download citation
DOI : https://doi.org/10.1007/978-1-84882-046-3_41
Publisher Name : Springer, London
Print ISBN : 978-1-84882-045-6
Online ISBN : 978-1-84882-046-3
eBook Packages : Medicine Reference Module Medicine
Share this entry
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Healthy Pet Checkup
Pacemakers for Dogs with Heart Problems

Human medicine has paved the way for several treatments and procedures that can be used similarly in cats and dogs. Thanks to this, many pets have been able to survive complex health conditions such as those involving the heart. In fact, for several years, it has been possible to install pacemakers in dogs, a device that helps regulate their heart rate. Do you want to know if your canine needs a pacemaker or is a good candidate to receive one? Keep reading to learn more!
Book a video consultation with an experienced veterinarian within minutes.
- Professional vet advice online
- Low-cost video vet consultations
- Open 24 hours a day, 365 days a year
What are pacemakers?
A pacemaker is a device that, through surgical intervention, is integrated into the dog's heart to help it maintain a beating frequency appropriate to its needs. It consists of a pulse generator (battery), an electrode that connects it to the heart's myocardium, and programming equipment.
Its use is better known in humans, but as we indicated, pacemakers can also be placed in dogs whose heart beats too slowly. This condition is known as bradycardia and carries a high risk of death, as we will explain below. The device will ensure that the heart does not slow down or stop again, thus extending both the quality and the life expectancy of your dog.
Bradycardia and Diseases that Require Pacemakers for Dogs
To understand bradycardia, we can start with the fact that the dog's heart is equipped with its own natural pacemaker called the sinus node . Its function is basically what we explained for the pacemaker: to create electrical impulses transmitted to the heart to produce the much-needed beats.
In some cases, the beats can slow down, leading to a low heart rate, or even stopping altogether. This can indicate failures in the natural pacemaker or the electrical impulse transmission to the rest of the heart, for example, in some part of the "wiring." Each of these cases that require pacemakers for dogs has a name: sinus node disease or sick sinus syndrome and heart block, respectively.
Sick Sinus Syndrome (SSS)
SSS occurs when the natural pacemaker, the sinus node, malfunctions, causing the dog's heartbeat to become irregular. These can be both fast and very slow. They usually occur in West Highland White Terriers, Dachshunds, Boxers, Cocker Spaniels, Miniature Schnauzers, and Standard Schnauzers.
Atrial Block
This condition is much rarer than SSS. An atrial block consists of the absence of electrical and mechanical activity in the atria, the two upper chambers that receive the blood that enters the dog's heart. A more severe form known as advanced atrioventricular block may also develop.
Signs Your Dog May Need a Pacemaker
With so many technical terms, caregivers are probably still at a loss about whether their dog may need a pacemaker. For clarity, it's worth discussing symptoms that often suggest something is wrong with a dog's heart.
The symptoms that can indicate a heart problem include weakness, lethargy, fainting , shortness of breath and increased respiratory rate while at rest, exercise intolerance, and decreased appetite.
The diagnosis of sick sinus syndrome or atrial block and the need for a pacemaker will depend on recommendations from a veterinary cardiologist. After a physical evaluation of your canine patient, your vet could recommend tests to confirm or rule out the possible causes of the symptoms mentioned.
The cardiac study will include at least one electrocardiogram , accompanied by detailed blood tests . The vet may also order chest x-rays and an abdominal ultrasound if any systemic disease, such as cancer, is suspected.
It is worth noting that canine candidates for a pacemaker are those in which the bradycardia is not due to structural heart disease. Most canines weighing more than 3 kg can receive this device through a procedure known as transvenous pacemaker implantation; for those with less weight, a different procedure is used.
Risks of Dog Pacemaker Surgery
Dog pacemakers are a very effective solution to the problems mentioned above, but it is an invasive procedure, and the simple fact of requiring anesthesia increases the risks for patients.
For example, in dogs where the heart rate is very low, the effects of anesthesia can be fatal. The good news is that there are ways to reduce these scenarios, such as pacing the dog's heart before administering any of these anesthetic medications.
What is the prognosis for dogs after placing a pacemaker?
In general, the prognosis after the implantation of a pacemaker for dogs is very positive. However, it is necessary to maintain a series of care after the intervention to see results.
After the surgery, dogs usually return for follow-up every two days, and temporarily have their necks wrapped in a large bandage. The bandage’s function is essential and goes beyond protecting the device; it will prevent irritation, infection, and swelling of the incision site, as well as limit head movement.
This brings us to another crucial point - for four to five weeks, the canine's physical activity should be minimal to facilitate their wound healing. Therefore, jumping and interactions with other pets are discouraged. Collars will also be prohibited for the same reason, so using a harness is recommended instead.
Can dogs develop heart arrhythmias?
Atrial Fibrillation in Dogs and Cats
Ventricular Tachycardia in Dogs and Cats

Need to speak with a veterinarian regarding your dog’s heart problem or another condition?
Click here to schedule a video consult to speak to one of our vets. You can also download the FirstVet app from the Apple App Store and Google Play Stores.
More articles about Dog
Dog ownership costs from head to tail.
Puppies are irresistibly cute! But their unrelenting energy can tax your patience and make a mess.…
Pre-Existing Conditions: Helpful Tips You'll Appreciate About Pet Health Insurance
Pre-existing conditions are health concerns, such as diabetes, kidney disease, or an oral…
Discospondylitis in Dogs
Infections are one of the most common conditions among humans and their pets. Some of them can be…
Are you concerned about your pet?
- +1 312-757-6146
- [email protected]
About FirstVet
- Meet the vets
- How it works
- Terms and Conditions
- Privacy Policy
- Informed consent
- Accessibility Statement
With FirstVet, the vet clinic and pet shop are only one tap away. Get fast advice, trusted care and the right pet supplies – every day, all year round.
FirstVet Inc
900 3rd Ave 29th Floor
- Research article
- Open access
- Published: 27 March 2018
A retrospective review of 146 active and passive fixation bradycardia lead implantations in 74 dogs undergoing pacemaker implantation in a research setting of short term duration
- Lynne E. Swanson ORCID: orcid.org/0000-0002-2177-4465 1 ,
- Barbara A. Huibregtse 2 &
- Brian A. Scansen 3
BMC Veterinary Research volume 14 , Article number: 112 ( 2018 ) Cite this article
3869 Accesses
6 Citations
2 Altmetric
Metrics details
Canine veterinary patients increasingly benefit from implantation of transvenous pacemakers for bradyarrhythmias. No published data exist examining procedural outcomes of pacemaker implantation performed in the preclinical laboratory. The purpose was to review short term complication, infection, dislodgement, penetration rates, plus overall morbidity following pacemaker implantation in the research setting. A retrospective review of 74 Class A purpose-bred mongrels implanted with active ( n = 89) and passive fixation ( n = 57) intracardiac leads for dual ( n = 72) or single ( n = 2) chamber pacing was performed.
All leads were implanted successfully, meeting electrical implant criteria. Follow-ups typically occurred every 7 days (first month), then at 30 day intervals. Seroma formation was 1.4% and 10.8% at the venotomy and pulse generator site respectively. Overall infection rate was 1.4%. Overall dislodgement rate was 2.1%, (2 passive atrial leads, 1 passive ventricular lead). Overall fractures and insulation defects were zero. Two helix penetrations were noted incidentally post mortem, one at the right atrial appendage and one at the right ventricle (64 dogs, 128 leads evaluated), a 1.6% event rate. Major in-life adverse events were 5.4% (4 of 74 dogs), including 1 infection and 3 lead dislodgements.
Conclusions
This review demonstrates a low complication rate with bradycardia lead implants in the short term (up to 180 days), in a high volume research setting. Lead type, implant technique, surgeon experience, healthy patient population, patient size and follow-up care play a role. This review also suggests active fixation leads in the right atrial appendage of dogs are safe and reliable.
Introduction
Pacemaker and lead implantation procedures have been performed within the veterinary field for over 40 years, with the first implant in 1967 via a thoracotomy and epicardial placement of 2 leads for fixed rate pacing of 70 bpm [ 1 ]. Most veterinary patients undergoing pacemaker implantation today receive transvenous lead implants, resulting in a minimally-invasive procedure [ 2 , 3 , 4 , 9 , 14 ]. Advances in technology by the medical device industry have resulted in a wide range of active and passive transvenous pacing leads for human use that the veterinary clinician can now choose from for their specific patients, with refined smaller profiles, flexible lead bodies, different fixation types and insulation materials. Advances have also been made with pulse generators (PG), now smaller in size, with longer battery lives, offering a variety of software choices and device features for diagnostic and therapeutic programming. Such advances can pave the way for how the veterinary field manages their own patients in need of this therapy. Lower profile leads and smaller PGs allow for application of transvenous pacing to very small canine patients, who in the past may have had to undergo surgical placement of epicardial leads, but may now undergo a minimally invasive procedure. In tandem with these advances, implantation techniques have been refined in the preclinical setting with the ability to implant one, two, or even up to three leads in canine patients via a minimally invasive approach. All prototypes and iterations of pacing technology begin in the preclinical research realm, so the sharing of best research practices with the clinical veterinary community regarding lead types, implant techniques, and lead performance in terms of acceptable electrical criteria and device programming may facilitate transfer of knowledge for the betterment of veterinary clinical practice and veterinary patients.
Similar to human use, veterinary patients with symptomatic bradycardia, variations of atrioventricular (AV) block and sinus node dysfunction (SND) benefit from this device therapy. Breeds including west highland white terriers, miniature schnauzers and cocker spaniels tend to be predisposed to SND, while breeds such as Labrador retrievers and German shepherds appear predisposed to AV block [ 2 , 3 ].
Published data have documented the clinical complications from lead implants exhibited in these veterinary patient populations. Overall rates for major complications consisting of lead dislodgement, infection, cardiac arrhythmias leading to death, battery issues, or programming errors range from 9 to 33% [ 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 ]. There are also clinical cases of cranial or caudal vena caval syndrome related to transvenous pacemaker implantation [ 16 , 17 , 18 , 19 , 20 ]. No published data exist, however, examining the procedural and post-operative outcomes expected in the short term, under ideal settings, in a healthy patient population, within a preclinical laboratory environment. Data on pacemaker complications in such an idealized setting may provide a useful comparison for future clinical veterinary studies. Therefore, the aim of this study was to characterize the canine population, lead types, surgical procedures, peri- and post-operative processes, in-life events and gross pathology in a research laboratory with several decades of experience implanting transvenous pacemakers in dogs.
Materials and methods
All studies were reviewed by our Institutional Animal Care and Use Committee, with full approval and consent of all Class A research animals in accordance with the polices and guidelines of our institution, which is an AAALAC International accredited facility. All studies complied with animal use regulations as set forth in the United States Department of Agriculture Animal Welfare Act, 9, CFR, and adhered to the principles outlined in the "Guide for the Care and Use of Laboratory Animals," from the National Research Council.
Data were collected retrospectively from 71 dogs, from 2005 through 2016 with study durations of 42 to 180 days (29.6% 42–90 days and 70.4% 162–180 days) from complete data sets that were audited for FDA submission. An additional 3 dogs, that had implant durations of over 5 years ( n = 3), were included after reviewing electronic animal records, written documentation, radiographs and other electronic media archived within 2 additional study protocols. A total of 2 single chamber device systems and 72 dual chamber device systems were implanted. The inclusion of animals from a study was based on specific lead construction type and PG device implanted. All 146 leads evaluated were bradycardia leads and the majority (61.0%, 89 leads) were of the active fixation helix lead type, of which 22.5% (20 leads) were open helix type and 77.5% (69 leads) were extendable/retractable type. The remaining implants consisted of passive leads with silicone tine fixation (39.0%, 57 leads). Animals were chosen for this review based on the use of lead types and PG devices comparable to those implanted clinically in veterinary patients. Implantable cardioverter-defibrillator and cardiac resynchronization devices were excluded. The lead models were 6 Fr to 7Fr bipolar silicone or polyurethane endocardial pacing leads for use in the right atrium (RA) or right ventricle (RV). The passive models had silicone rubber tines with a silicone rubber collar at the distal tip containing 1.0 mg dexamethasone acetate to reduce pacing thresholds after acute implantation injury. Footnote 1 The active fixation models consisted of an open cork-screw helix coated with a mannitol tip Footnote 2 or an extendable-retractable helix allowing for mapping capability prior to fixation with radiopaque markers allowing fluoroscopic visualization of full extension. Footnote 3
Inclusion criteria
All dogs were purpose-bred mongrel dogs and had been evaluated prior to approval and enrollment into any study. This pre-screening evaluation consisted of a physical exam, clinical pathology, heartworm status, ECG analysis and parasite screen. To qualify for inclusion, the dogs had to exhibit no external signs of health issues, normal blood parameters, negative heartworm status, negative result on fecal floatation and normal cardiac rhythm and morphology used to screen for chamber enlargement, conduction disturbances, arrhythmias, abnormal repolarization/depolarization changes and signs of ischemia. Once all criteria were met , dogs were deemed healthy and considered to be acceptable for enrollment into a study.
Statistical comparisons were made between active fixation and passive fixation leads for the rate of dislodgment and the rate of perforation for both the RA and RV using commercially available software. Footnote 4 The Fisher’s exact test was used for statistical comparisons between groups. A P value < 0.05 was considered significant.
Preparation and anesthesia
All dogs were fasted a minimum of 12 h prior to surgery and were bathed with a medicated shampoo Footnote 5 within 24 h prior to implant.
Because of the retrospective nature of this review, there were slight alterations of anesthetic regimens and follow-up intervals. In general, each dog was sedated with 10 mg of IM butorphanol. Preventative antibiotics were given approximately 30 min prior to any incision and consisted of cefazolin Footnote 6 (~ 30 mg/kg), given IV at the induction of general anesthesia. General anesthesia was induced with 200 mg of IV ketamine and 10 mg IV diazepam or 1 to 6 mg/kg of IV propofol. A surgical plane of anesthesia was maintained using isoflurane gas. After induction, the entire dorsal, lateral and ventral neck region encompassing the right side from the base of the head to the cranial edge of the scapulae was clipped and aseptically prepared for surgery. During implant, analgesia was maintained throughout the procedure with 10 mg of IV butorphanol every 1 to 1.5 h until the surgical portion was complete.
All dogs were placed on a ventilator with rate and tidal volume adjusted as needed to maintain stable physiological parameters. During the procedure, IV fluids (~ 5 ml/kg/h) with the vasopressor phenylephrine at a constant rate infusion (2–10 μg/kg/min) were commonly infused to maintain intravascular pressure and reduce total administered fluid volume.
All transvenous lead implants were performed by one of four dedicated veterinary interventionalists within the research facility with extensive experience in lead implantation. Each interventionalist was specifically trained on lead handling techniques and pacemaker implantation in dogs, pigs and sheep. Appropriate implant location was confirmed by meeting specific electrical criteria at implant, followed by appropriate pacing and sensing during the lead maturation period (typically the first 30 days), and subsequently, throughout the entire study duration. Full details of the implant procedures and post-implant animal care can be found in Additional file 1 .
Outcomes were based on short term implantation of device systems ranging from approximately 2–6 months in duration, in an ideal environment, with dogs free of morbid cardiovascular conditions or other comorbidities. Class A mongrel dogs ranged in age from 5 to 27 months and in weight from 20.0 to 28.0 kg at time of implant. Data from 74 dogs and 146 lead implants were collected. Of the 146 leads evaluated, 28 were passive and 44 were active fixation leads implanted into the RA; 29 were passive and 45 were active fixation leads implanted into the RV. Of the 44 active fixation leads in the RA, 22.7% (10 leads) consisted of open helix leads and 77.3% (34 leads) were extendable/retractable helix lead types. Similarly, of the 45 active fixation leads in the RV, 22.2% (10 leads) were open helix leads and 77.7% (35 leads) were the extendable/retractable helix lead types.
In recovery, there were no adverse events recorded in any dog. Post-operatively, there were 3 dislodgements, all passive fixation leads (2.1% of all implants). Two preformed passive J leads dislodgements from the RA were noted on day 4 radiographs and 1 passive straight lead dislodgement from the RV was noted on day 7 radiographs (Table 1 ).
Overall infection rate was 1.4% (1 dog) across all 74 dogs. This dog exhibited seromas involving both the device pocket and venotomy site, with intermittent drainage and positive growth of staphylococcus on culture of the blood and site. Due to the short 90 day duration of implant for the study protocol this dog was assigned to, because the dog was healthy, and to avoid the risk of additional anesthesia and device explantation, the dog was maintained on antibiotics and anti-inflammatories throughout study duration. The dog remained bright, alert and active throughout the 90 day study period.
None of the studies reported any lead fracture, either during the in-life portion or upon examination post mortem. Electrical performance and function of all lead implants were deemed acceptable and fell within the accepted criteria at implant in unipolar and/or bipolar configurations (Table 2 ). Subsequently, leads did not exhibit any failure to sense or pace during the full in-life period, with the exception of the three dislodgements.
Excluding lead dislodgements and the infection noted above, there were no major adverse events, either during a follow up procedure or noted clinically during the in-life period in any of the 74 dogs. A major adverse event was defined as any in-life device related death, lead perforation or fracture, clinical event requiring life-saving therapeutic intervention such as pneumothorax, or any incident requiring additional surgery. A follow up was defined as a procedure that required sedation for radiographs or fluoroscopic imaging to monitor chronic lead fixation positioning as well as collection of electrical data with a programmer to measure unipolar and/or bipolar lead performance in specific pacing modes and programmable parameters; follow up events also included sedation plus anesthesia in order to perform extensive data collection on the test system performance, such as hemodynamic and electrical data before, during and after an MRI scan.
A post mortem necropsy was performed on 65 of the 74 dogs reviewed (3 still alive; 6 transferred to a separate study), consisting of 130 lead sites. Of the 130 implant sites, there were 2 helix penetrations of the epicardium observed, one active fixation extendable/retractable lead type in the RA and one active fixation extendable/retractable lead type in the RV, a 1.5% event rate (Fig. 1 ). These were incidental findings at necropsy only, the dogs not having exhibited any clinical signs indicative of a possible lead perforation (i.e. pneumothorax, pericardial effusion, focal lung lobe radiopacity, mediastinal changes etc.) either clinically or on serial radiographs and bloodwork. Variable encapsulation of the distal portion of the lead body was observed, as commonly noted with long term implants (Fig. 2 ). There were no other abnormal findings on the remaining 65 dogs at the RA or RV lead/tissue interface.
Typical gross presentation of RA appendage ( a ) and RV ( b ) lead helix penetration
Variations on chronic mature endocardial lead implant sites. a RA appendage, active fixation lead, 90 days post implant; ( b ) RA appendage, active fixation lead, 47 days post implant; ( c & d ) Both right ventricles, active fixation leads, 180 days post implant
To the authors’ knowledge, this is the first time data has been collectively analyzed from a retrospective examination of pacemaker lead implantation performed in the preclinical laboratory setting. It should be emphasized that the preclinical research environment is well controlled, and studies are performed to show safety, not efficacy, for the regulatory agencies. Overall outcomes are based on evaluations in the short term, in overtly healthy patients. Complication rates in this review are therefore not directly comparable to real life clinical studies, due to the very different patient populations and co-morbidities found in clinical veterinary patients. However, the data may prove useful to veterinary cardiologists as baseline expectations for short term complications in near perfect conditions with young, healthy patients. Clinical management practices learned in the research laboratory may have applicability to practitioners who implant pacemakers in clinical canine patients. With the caveats noted above in mind, we did find some differences in complication rates between the data acquired in the study as compared to what has been documented in the literature gathered from the clinical canine setting. The reported rate of lead dislodgement in clinical patients varies from 6% [ 4 ] to 10% [ 7 ], with a value of 2.1% reported here. Reported infection rates for clinical veterinary patients vary from 1% [ 3 , 14 ] to 6% [ 4 ], with 1.3% reported here. Reporting of the total number of major complications varies between studies, but includes reports of 9% of 104 dogs [ 14 ], 10% of 105 dogs [ 3 ], 23% of 136 dogs [ 7 ], and 27% of 33 dogs [ 4 ]; the total major in-life complication rate reported here was 5.4% for 74 dogs (1 infection, 2 RA appendage dislodgements, and 1 RV lead dislodgement).
Dogs are the model of choice for this type of preclinical research because of similar cardiac structure, function and size to that of humans. Much of the prior knowledge and experience related to bradycardia, tachycardia and pacemaker devices have been carried out using the in vivo canine model and this model is well characterized and well accepted by the FDA. Swine are a less useful model due to their growth dynamics and the historical observations that swine react differently to subcutaneous and sub muscular device implants, with a high incidence of abnormal reaction to foreign bodies, resulting in PGs eroding through the skin. There are several factors that may explain the lower rate of complications in this review. All study protocols for transvenous lead systems for this device company were implanted in larger (> 18 kg), USDA Class A origin, with little to no variability. This population inherently decreases the risk of inadequate vessel or chamber size for implantation and allows for appropriate lead slack, a more difficult procedure in smaller dogs in the real world setting. These research animals have no pre-existing disease and veterinarians or surgical research specialists working within a cardiovascular medical device company have the luxury of gaining considerable experience in lead implantation by sheer numbers alone, as that is the focus of much of the business development and innovation. Because of this, refinement of technique in lead site selection, tunneling, lead slack optimization, suture sleeve positioning and tightening, device pocket sizing and securement and being able to troubleshoot with electrical data challenges is easily achievable. Although nearly all the implants in this review were dual chamber device systems, which have comparable complication rates to single chambered systems in the veterinary field [ 4 , 6 ], together, lack of patient morbidity, short to medium-term duration of implant, and operator experience likely play a large role in the incidence of complications, which is similar to what has been documented in human medicine [ 21 ]. Lastly, these animals are not discharged, but closely monitored on a daily basis, with highly technical human resources playing a big role in their aftercare. These animals are in a very well controlled environment, with close supervision on exercise restriction, daily observation of incision sites and daily bandage changes to insure constant pressure at the device pocket over a 2 week period, all which aid in limiting complication rates.
Descriptions of these procedures and processes, acquired from decades of research on preclinical canine pacemaker implantation, to share with the clinical veterinary cardiology community are provided in Additional file 1 . Lead slack redundancy appears to be the most common difference between preclinical techniques and reports from the clinical veterinary field. In human medicine, and in images from clinical veterinary reports [ 3 , 14 ], lead bodies positioned in either the atrium or ventricle are implanted with little slack, which is considered adequate. This is logical for humans due to the bipedal stance and passive disposition of a typical cardiac implant patient. For dogs however, adequate slack is defined differently, with a gentle “S” curve (Fig. 3 ) required for the ventricular lead, to mitigate the quadruped stance, the changing dynamics, physics and length of the neck, and their spirited disposition. Too little slack, visualized as a straight line out the tricuspid valve, increases the risk of dislodgement once the dog is awake because of the lack of accommodation for neck and body movement. For the atrial lead, there should be enough slack such that the lead body rests just above the tricuspid valve annulus (Fig. 3 ). Too little slack, or an “L” shape, can result in too much tension on the helix and increases the risk of dislodgement. Too much slack in the lead body impinges on the tricuspid valve and could also result in excessive force against the helix tissue interface and increase the risk of myocardial penetration. Of potential rare complications that can occur, asymptomatic perforation is a known phenomenon and in most cases does not result in electrophysiologic consequences [ 22 ]. Passive lead perforation was not noted in this review, but has been documented clinically 7 weeks after implantation into the RV apex [ 23 ]. Implementing a small change in slack can result in large reductions in lead dislodgements. Although this is likely much more challenging due to smaller chamber sizes in the smaller patients that are a significant proportion of the patient population seen clinically in veterinary cases, slack initiatives should be implemented to help limit dislodgement.
Standard slack allowance for RA and RV lead implants
An understanding of lead type construction and fixation properties is also important. This review demonstrated that both active and passive lead types appear safe and reliable for implantation in either chamber. There was no statistical difference between the two groups (active vs. passive) in the incidence of dislodgement or perforation (Table 1 ); however, the study was not powered for this endpoint.
This review also suggests that an active fixation lead implanted into the RA appendage (44 implants in this review) by experienced operators using appropriate techniques is safe in the short term and effective (in terms of lead electrical performance). Such an approach has not been fully utilized clinically, due to the perception that an optimal lead design for veterinary patients does not yet exist, that the thin walled RA appendage will not necessarily allow for proper fixation into the tissue, the presumption that there is a greater risk of perforation, and recognition of the need to develop optimized techniques to make atrial pacing more reliable [ 5 , 24 ].
Device safety is mandated by regulatory agencies and the research setting plays an important role assuring that these devices and the procedures associated with their implantation are safe and translatable in improving human health. Therefore, many studies in an animal model are performed to confirm safety with the advent of improved technologies resulting in refined leads and pulse generators. However, it is understood that a systemic review of relevant research studies in this setting does not exhibit the same diverse population, nor requirements of long term efficacy encountered in real life for practicing veterinarians. Interpretation of these results needs to be taken in context, understanding they are from data of short duration, in healthy patients, with little heterogeneity. With the above caveat, this review demonstrates a low complication rate with bradycardia lead implants in the short term (up to 180 days), in a high volume research setting. This review also suggests active fixation leads in the right atrial appendage of dogs are safe and reliable.
Limitations
A direct comparison to clinical results in the veterinary field is limited in that these preclinical studies are short term in nature, with implants into overtly healthy patients. These animals are housed and cared for in a controlled environment with daily professional care for the full duration of implant. In addition, the volume of implants performed in a medical device company research facility is presumably higher than in the real life clinical setting, resulting in greater operator experience. The dogs are young and deemed healthy upon enrollment into every study, without any competing morbid condition. The dogs are also of larger size, all > 18 kg. Cranial or caudal vena caval syndrome is not recognized in this patient population, likely due to the larger size of preclinical dogs and therefore this review does not provide a representative sampling of complication rate secondary to single or multiple lead implantation. Lastly, duration of implant is dictated by FDA guidelines for safety requirements and the necessity for device/tissue interface data; the presence of only 3 dogs with long term outcomes (> 2 years) in this study prevents an analysis of long term complication rates that may be expected in the preclinical setting. It should also be noted that the definition of seroma in this review varied in documentation from minimal to moderate in size, but all incidences were counted, which may have skewed the data to a higher event rate than what is reported in clinical cases. Electrical data was taken to insure optimal location and device contact with the myocardium at implant and lead performance was confirmed by testing appropriate behavior in terms of sensing and pacing over the duration of the study, but data was not collected to assess efficacy. Despite these limitations, this review provides data on the expected complication rate of pacemaker implantation in the dog under idealized circumstances and suggests that the placement of active fixation leads in the RA of dogs is both safe and reliable in the short term.
FINELINE™ II Sterox Atrial-J and straight silicone and polyurethane passive fixation pacing leads, Guidant Corporation, St Paul, MN; INGEVITY™ passive fixation Atrial-J and straight polyurethane pacing leads, Boston Scientific, St. Paul, MN
FINELINE™ II Sterox EZ, silicone and polyurethane active fix pacing lead, Guidant Corporation, St. Paul, MN
INGEVITY™ pacing lead, Boston Scientific, St. Paul, MN; DEXTRUS™ pacing lead, Guidant Corporation, St. Paul, MN
Minitab (version 17.1.0), Minitab Inc., State College, Pennsylvania
PYOBEN® Medicated Shampoo, Virbac Corporation, Fort Worth, Texas
Cefazolin, WG Critical Care, LLC, Vernon Hill, Illinois; West Ward Pharmaceutical Corporation, Eatontown, New Jersey
Abbreviations
Atrioventricular
Code of Federal Regulations
Food and Drug Administration
Pulse generator
Pulse width
Right atrium/atrial
Right atrial appendage
Right ventricle/ventricular
Buchanan JW. First pacemaker in a dog: a historical note. J Vet Intern Med. 2003;17:713–4.
Article PubMed Google Scholar
Sisson D, Thomas WP, Woodfield J, et al. Permanent transvenous pacemaker implantation in forty dogs. J Vet Intern Med. 1996;5(6):322–31.
Article Google Scholar
Wess G, Thomas W, Berger D, et al. Applications, complications, and outcomes of transvenous pacemaker implantation in 105 dogs (1997-2002). J Vet Intern Med. 2006;20:877–84.
PubMed Google Scholar
Hildebrandt N, Stertmann WA, Wehner M, et al. Dual chamber pacemaker implantation in dogs with atrioventricular block. J Vet Intern Med. 2009;23:31–8.
Article CAS PubMed Google Scholar
Estrada AH, Pariaut R, Hamsley S, et al. Atrial-based pacing for sinus node dysfunction in dogs: initial results. J Vet Intern Med. 2012;26:558–64.
Genovese D, Estrada AH, Maisenbacher H, et al. Procedure times, complication rates, and survival times associated with single-chamber versus dual-chamber pacemaker implantation in dogs with clinical signs of bradyarrhythmia: 54 cases (2004-2009). JAVMA. 2013;242(2):230–6.
Oyama MA, Sisson DD, Lehmkuhl LB. Practices and outcome of artificial cardiac pacing in 154 dogs. J Vet Intern Med. 2001;15:229–39.
Ward JL, DeFrancesco TC, Tou SP, et al. Complication rates associated with transvenous pacemaker implantation in dogs with high-grade atrioventricular block performed during verses after normal business hours. J Vet Intern Med. 2015;29:157–63.
Article CAS PubMed PubMed Central Google Scholar
Domenech O, Santilli R, Pradelli D, et al. The implantation of a permanent transvenous endocardial pacemaker in 42 dogs: a retrospective study. Med Sci Monit. 2005;11(6):168–75.
Google Scholar
Fine DM, Tobias AH. Cardiovascular device infections in dogs: report of 8 cases and review of the literature. J Vet Intern Med. 2007;21:1265–71.
Darke PGG, McAreavey D. Been M. Transvenous cardiac pacing in 19 dogs and one cat. J of small animal. Practice. 1989;21(9):491–9.
Bonagura JD, Helphrey ML, Muir WW. Complications associated with permanent pacemaker implantation in the dog. J Am Vet Med Assoc. 1983;182(2):149–55.
CAS PubMed Google Scholar
Lichtenberger J, Scollan KF, Bulmer BJ, et al. Long-term outcome of physiologic VDD pacing versus non-physiologic VVI pacing in dogs with high-grade atrioventricular block. J Vet Cardiol. 2015;17(1):42–53.
Johnson MS, Martin MWS, Henley W. Results of pacemaker implantation in 104 dogs. J Small Animal Practice. 2007;48(1):4–11.
Article CAS Google Scholar
Coleman AE, DeFrancesco TC, Chanoit G. Pacemaker malfunction due to mechanical failure of the lead-header interface. J Vet Cardiol. 2012;14(4):519–23.
Murray JD, O’Sullivan ML, Hawkes KC. Cranial vena caval thrombosis associated with endocardial pacing leads in three dogs. J Am Anim Hosp Assoc. 2010;46(3):186–92.
Van De Wiele CM, Hogan DF, Green HW III, et al. Cranial vena caval syndrome secondary to transvenous pacemaker implantation in two dogs. J Vet Cardiol. 2008;10(2):155–61.
Stauthammer C, Tobias A, France M, et al. Caudal vena cava obstruction caused by redundant pacemaker lead in a dog. J Vet Cardiol. 2009;11(2):141–5.
Cunningham SM, Ames MK, Rush JE, et al. Successful treatment of pacemaker-induced stricture and thrombosis of the cranial vena cava in two dogs by use of anticoagulants and balloon venoplasty. J Am Vet Med Assoc. 2009;235(12):1467–73.
Mulz JM, Kraus MS, Thompson M, et al. Cranial vena caval syndrome secondary to central venous obstruction associated with a pacemaker lead in a dog. J Vet Cardiol. 2010;12(3):217–23.
Eberhardt F, Bode F, Bonnemeier H, et al. Long term complications in single and dual chamber pacing are influenced by surgical experience and patient morbidity. Heart. 2005;91(4):500–6.
Hirschl DA, Jain VR, Spindola-Franco H, et al. Prevalence and characterization of asymptomatic pacemaker and ICD lead perforation on CT. PACE. 2007;30:28–32.
Achen SE, Miller MW, Nelson DA, et al. Late cardiac perforation by a passive-fixation permanent pacemaker lead in a dog. J Am Vet Med Assoc. 2008;233(8):1291–6.
Jones AE, Estrada AH, Pariaut R, et al. Atrial septal pacing in small dogs: a pilot study. J Vet Cardiol. 2014;16(3):163–71.
Download references
Acknowledgements
The authors gratefully acknowledge David F. Warnock and Linda Hansen for technical assistance and manuscript review.
All studies were funded internally by the cardiac rhythm management division within Boston Scientific Corporation.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available but data may be available from the corresponding author, on reasonable request, with appropriate justification, after signing a confidential disclosure agreement with the institution.
Author information
Authors and affiliations.
Research and Technology Center, Boston Scientific Corporation, 4100 Hamline Ave North, Saint Paul, MN, 55112, USA
Lynne E. Swanson
Preclinical Sciences, Boston Scientific Corporation, 100 Boston Scientific Way, Marlborough, MA, 01752, USA
Barbara A. Huibregtse
Department of Clinical Sciences, Colorado State University, 1678 Campus Delivery, Fort Collins, CO, 80523-1678, USA
Brian A. Scansen
You can also search for this author in PubMed Google Scholar
Contributions
LES contributed the concept and design of the work, acquisition and interpretation of the data and writing of the manuscript; BAH contributed to the concept and scope of the manuscript, as well as review and editing; BAS contributed to the conception, critical review and editing of the manuscript for intellectual content. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Lynne E. Swanson .
Ethics declarations
Ethics approval and consent to participate.
All studies were approved by the Institutional Animal Care and Use Committee, Boston Scientific Corporation, with full approval and consent of all Class A research animals in accordance with the policies and guidelines of our institution, which is an AAALAC International accredited facility. All studies complied with animal use regulations as set forth in the United States Department of Agriculture Animal Welfare Act, 9, CFR, and adhered to the principles outlined in the "Guide for the Care and Use of Laboratory Animals," from the National Research Council.
Consent for publication
Not applicable
Competing interests
Both Lynne Swanson and Barbara Huibregtse are employees of Boston Scientific Corporation, who funded all studies reviewed within this manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:.
Implantation Procedures and Follow-up Care. Didactic description of the procedures and processes for intracardiac atrial and ventricular lead implantation and follow-up care in the research setting. (ZIP 303 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Swanson, L.E., Huibregtse, B.A. & Scansen, B.A. A retrospective review of 146 active and passive fixation bradycardia lead implantations in 74 dogs undergoing pacemaker implantation in a research setting of short term duration. BMC Vet Res 14 , 112 (2018). https://doi.org/10.1186/s12917-018-1431-2
Download citation
Received : 23 October 2017
Accepted : 15 March 2018
Published : 27 March 2018
DOI : https://doi.org/10.1186/s12917-018-1431-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- transvenous leads
- complications
BMC Veterinary Research
ISSN: 1746-6148
- General enquiries: [email protected]

- Publications
ECG reading session-cardiac arrhythmias (Proceedings)
Arrhythmias can be classified based on ECG analysis based on the heart rate (normal, bradyarrhythmias, tachyarrhythmias); anatomic origin of the rhythm disturbance (SA, atrial, atrioventricular, or ventricular); or electrophysiologic mechanism when evident.
Arrhythmias can be classified based on ECG analysis based on the heart rate (normal, bradyarrhythmias, tachyarrhythmias); anatomic origin of the rhythm disturbance (SA, atrial, atrioventricular, or ventricular); or electrophysiologic mechanism when evident. Keys to recognizing cardiac arrhythmias include an analysis of rate, regularity, patterns, P-QRS relationship, waveform morphology, and conduction intervals. In terms of a methodological approach to rhythm diagnosis, it is recommended that one begin as follows: 1) Identify the patient, lead(s), paper speed, calibration signals, and artefacts; 2) Decide if the rate is slow, normal, or fast for the species; 3) Identify regularity or lack thereof and search for repetitive patterns in irregular rhythms; 4) Identify P and QRS complexes and the relationship between these waveforms; 5) Scrutinize the morphology and consistency of the P-waves and the QRS complexes; 6) Consider the conduction intervals across the atria (P-wave duration), atrioventricular conduction system (P-R interval), ventricles (QRS duration), and overall repolarization time (Q-T interval); 7) Identify the frontal axis as normal, left, or right; 8) Evaluate the QRS morphology for conduction disturbances, obvious bundle branch or fascicular blocks, and for cardiomegaly pattern(s); 9) Assess the ST-T for repolarization abnormalities; and 10) interpret the ECG with consideration of the entire clinical and laboratory picture.
Sinus rhythms
Physiologic rhythms during routine exam include normal (regular) sinus rhythm and sinus arrhythmia. Sinus rhythm disorders are often due to high vagal or sympathetic tone; any patient with sinus bradycardia or tachycardia should be evaluated with this in mind. Additionally, drugs, anesthetics, temperature, and endocrine status (thyroid or adrenal) can affect sinus node rate. Dogs with respiratory disease can show pronounced sinus arrhythmia with wandering pacemaker; the short cycles can resemble premature atrial complexes. Management of sinus rhythm disturbances is focused first on treating any underlying conditions. Occasionally inappropriate sinus tachycardia is treated with a beta-blocker. Sinus bradycardia can be treated in the hospital with atropine or glycopyrrolate. Chronic, progressive, sinus node dysfunction is common in miniature Schnauzers, West Highland white terriers, and cocker spaniels. Insufficient escape activity may result in collapse or syncope ("sick sinus syndrome"). The best long-term therapy for this syndrome is not drugs like anticholinergics or terbutaline but permanent transvenous pacing. Pacemaker programming is critical for optimal system performance (e.g. VVIR mode) and long-term outcomes are generally excellent.
Supraventricular arrhythmias
Supraventricular rhythm disturbances are among the most common and difficult of all ECG diagnoses. These arrhythmias include premature atrial complexes, atrial tachycardia, atrial flutter, atrial fibrillation (AF), re-entrant supraventricular tachycardia (SVT), and atrial standstill. Supraventricular arrhythmias can be transient, recurrent, or permanent. In most cases, recurrent or permanent arrhythmias are caused by structural heart diseases associated with congenital, chronic valvular, myocardial, or pericardial disease. Some giant canine breeds develop chronic atrial arrhythmias without overt structural disease. Lone AF in Irish wolfhounds is an example.
The ventricular rate response in a SVT is determined by the type of arrhythmia and AV conduction: the ventricular response can be slow or fast; regular or irregular. In high-sympathetic states, AV conduction of supraventricular arrhythmias can be very rapid, as with AF in the setting of congestive heart failure (CHF). Organized, regular SVT associated with atrial tachycardia, atrial flutter or re-entrant SVT often induce ventricular responses of 300 to 400 per minute! In 2:1 AV conduction of atrial tachycardia or flutter, the rate may suddenly double or half as the conduction ratio (P':QRS) changes. Subtle electrical alternans is a common finding in regular SVTs regardless of mechanism. This finding may help to separate a pathologic SVT from a "fast" sinus tachycardia (wherein alternans is uncommon). Supraventricular tachyarrhythmias also can be conducted with bundle branch block, and the resultant QRS complexes can be confused with PVCs or ventricular tachycardia (VT).
Recurrent atrial premature complexes or atrial tachycardia are often treated with drugs that suppress ectopic rhythms, including lidocaine (acutely), sotalol, and amiodarone. When efforts to suppress these ectopics fail, ventricular rate control is the goal. When atrial tachyarrhythmias are associated with CHF, digoxin is chosen first, but otherwise, diltiazem or a beta-blocker are more effective for rate control, and sometimes these drugs will convert the rhythm back to sinus. Combined therapy with digoxin and diltiazem is often used in chronic AF associated with CHF. Synchronized DC cardioversion of atrial flutter/fibrillation is another approach, particularly in dogs with lone atrial fibrillation. Amiodarone or sotalol are often prescribed after cardioversion to maintain sinus rhythm. These drugs should be continued for at least three months if possible to prevent reversion to atrial fibrillation; however, amiodarone therapy can lead to severe hepatotoxicity in dogs.
Reentrant SVTs employ circuits that develop at the micro and macro levels. The best characterized in dogs use a circuit involving the atria, AV node, and an accessory AV pathway that bypasses (or longitudinally separates) the AV conduction system. The tachycardia is often triggered by a sudden change in sinus cycle length, a premature atrial or ventricular complex. In most cases the circuit is "orthodromic"; down the AV node with an associated normal (narrow) QRS. Retrograde P'-waves may be identified in the ST segment (an R–P'). In some dogs periods of sinus rhythm are associated with ventricular pre-excitation, a helpful clue to the presence of an accessory pathway. Pre-excitation is characterized by a short PR interval and early ventricular activation (the delta wave) with wide QRS and T-waves. Management of reentrant SVT is done with drugs initially (diltiazem and procainamide can be tried), but referral to a specialist for catheter ablation of the accessory path is the best treatment.
Atrial standstill indicates that the atrial muscle is inexcitable. This condition is caused transiently by high serum potassium or persistently by atrial muscle disease or severe atrial dilation (in cats). In these cases, no P-waves are evident (atrial standstill) or very tiny, non-conducted or broad-low amplitude P waves are evident. Persistent standstill is most common in English Springer spaniels, but can also occur in larger retriever breeds. In cats apparent atrial standstill can be observed with severe forms of cardiomyopathy.
Ventricular arrhythmias
Arrhythmias arising in the ventricle parallel those of the atria in terms of nomenclature. But there are important differences: 1) the AV node need not be activated to generate a QRS complex, and 2) there is greater potential for sudden death if the rhythm degenerates to ventricular fibrillation or asystole.
Idioventricular "escape" complexes are rescue mechanisms for sinus node arrest or AV block and should not be suppressed. The typical idioventricular rhythm in the dog discharges at 20 to 40/minute, but in the cat the rate is much faster, approaching 130/minute in many cats with complete AV block. Premature ventricular complexes (PVCs, VPCs) arise early, and can be uniform or multiform in morphology. A fusion complex between a PVC and a sinus impulse also can create intermediate QRS forms and are less serious. Ventricular tachycardia (VT) can be "slow" or "fast"; paroxysmal or sustained (>30 secs); monomorphic or polymorphic; or rapidly varying in orientation (torsade de pointes). The ventricles also can flutter (producing sine waves), or fibrillate (a disorganized and lethal activation). In very sick animals or in those with CHF, death can occur from asystole, which is essentially ventricular standstill.
Clearly PVCs are among the most common rhythm disturbances. Causes include primary electrical or structural heart diseases, electrolyte and metabolic disturbances, autonomic imbalance, drugs, toxins, and the "usual suspects", such as splenic masses and gastric dilatation. It can be difficult to decide if PVCs are "clinically significant" or not, but the issue is important. For example, most cats with chronic ventricular ectopy have structural heart disease (cardiomyopathy) or an elevated serum troponin suggestive of active myocardial injury or myocarditis. A Doberman pinscher (at least one from North America) with PVCs on a routine ECG is likely to progress towards overt dilated cardiomyopathy. Furthermore, when an ECG demonstrates even a few PVCs in a Doberman pinscher that has collapsed or fainted, the risk of sudden cardiac death within the year is very high. Such information may prompt antiarrhythmic therapy, recognizing that there is no proof treatment will prolong life. Conversely, some boxers have PVCs for years without signs and are best assessed by history and ambulatory (Holter) ECG monitoring.
ECG diagnosis of PVCs or of VT is generally straightforward. In many cases a full workup including drug history, history of clinical signs (weakness, collapse or syncope), Echo findings, laboratory tests (CBC, chemistries, serum troponin-I), and abdominal ultrasound may be needed to determine the likely cause and significance. The Holter ECG can help assess the severity of ventricular arrhythmias, complexity of the complexes, and response to therapy. The absolute number of "normal" PVCs (not simply ectopics) per day is controversial, but in the author's (arbitrary) opinion >10/day in cats and >50/day in dogs should be considered abnormal. Day to day variation is common (up to ~85%) and this must be taken into account when considering both severity and "response" to any treatment.
Management of ventricular ectopic rhythms involves determining the most likely cause, advancing an educated guess about the clinical significance, considering the need for therapy, and possibly choosing one or more drugs. All antiarrhythmic drugs carry the potential for side-effects and worsening of the arrhythmia (proarrhythmia). Lidocaine remains the drug of choice for acute management, with IV procainamide, esmolol, magnesium salts, and amiodarone as back up treatments. For chronic therapy, sotalol is generally the best tolerated (except for negative inotropic effects in CHF), but it not always as effective as mexiletine plus sotalol, mexiletine plus atenolol, or amiodarone. Amiodarone deserves respect, especially in terms impairing liver function. Flecainide may be another potentially useful drug but overall experience in dogs is low and the drug can lead to "pro-arrhythmia" in humans and in experimental canine arrhythmias.
Conduction disturbances
In addition to sick sinus syndrome, persistent atrial standstill, and ventricular pre-excitation (each discussed above), conduction disturbances include the AV blocks; bundle branch blocks, and intraventricular conduction disturbances. The AV blocks are classified as first, second (Mobitz I, Mobitz IIA and IIB), and complete (third-degree block). Treatment of symptomatic AV blocks generally involves referral for permanent pacing. Single or dual chamber pacing systems can be used, depending on a variety of patient and technical factors. Long-term prognosis depends mainly on etiology of the bradyarrhythmia (best prognosis for SSS and AV block without other structural diseases and worst for persistent atrial standstill). Persistent bundle branch block or phasic aberrant ventricular conduction can be encountered in structurally normal hearts or in those with disease of the bundle branches.
Antiarrhythmic therapy – overview
Drugs, electrolytes, pacing, direct current shock, and radiofrequency energy can be used to treat disorders of heart rhythm. Antiarrhythmic drug dosing varies and drug safety is highly dependent on ventricular function and proarrhythmic effects. General canine dosing guidelines are indicated below:
- Amiodarone (5 mg/kg IV as a slow infusion with caution (may want to pretreat with benadryl and dexamethosone to reduce risk of anaphylactoid reaction); 8 to 10 mg/kg PO daily x 2 weeks; thereafter 4–6 mg/kg PO daily); beware: cholestatic liver injury with related anorexia, vomiting and weight loss; thyroid dysfunction
- Atenolol (0.5–1 mg/kg PO q12h; reduce dosage at least 50% in congestive heart failure)
- Diltiazem (0.1 mg/kg slow IV boluses repeated to 0.4 to 0.5 mg/kg cumulative dose with BP monitoring; 3–6 mg/kg PO, total daily dose, divided b.i.d. or t.i.d. depending on the preparation. Initial doses in congestive heart failure should be lower – 1.5 mg/kg total daily dose – and then can be rapidly uptitrated)
- Esmolol (50–200 micrograms/kg/minute IV infusion); use care with anesthesia or LV dysfunction.
- Flecainide (1–2 mg/kg b.i.d. to t.i.d. – preliminary dose); negative inotrope so use with care in CHF; may widen QRS complex leading to proarrhythmia.
- Lidocaine (2 mg/kg boluses IV to 8 mg/kg cumulative dose; thereafter, 25–75 micrograms/kg/min constant rate infusion); beware: vomiting and neurotoxicity
- Mexiletine (5–8 mg/kg PO q8h) – can be combined with a beta blocker or sotalol; beware: anorexia and neurotoxicity; rarely acute hepatic injury; some oral preparations can no longer be obtained in USA.
- Procainamide (2 mg/kg slow IV boluses to 20 mg/kg cumulative dose; 10–20 mg/kg PO q8h of long-acting preparation); beware: hypotension; oral preparations have been discontinued in USA.
- Sotalol (1–2 mg/kg PO q12h); higher dosages may be tolerated in some dogs; beware: bradycardia
Effective use of any antiarrhythmic drug depends on clinical response and experience. Hypokalemia and hypomagnesemia can nullify the beneficial effects of class I agents while exacerbating pro-arrhythmic effects of Class III agents. Some drugs are more effective at particular heart rates (use dependence) and may worsen an arrhythmia by changing the rate. Every drug used to treat arrhythmias is considered an extralabel drug use in veterinary practice and treatment recommendations are based mainly on clinical experience; specific recommendations have been included above. Additional effects from activation/block of the autonomic nervous system and depression of myocardial contractility may occur. Most antiarrhythmic drugs demonstrate a proarrhythmic effects in a percentage of patients. Even beta-blockers can be proarrhythmic indirectly by slowing heart rate and prolonging cell cycle length. Thus antiarrhythmic therapy a true risk: benefit proposition.

Top Vet Blast Podcast episodes of 2023: #9
We have compiled a countdown of our top Vet Blast Podcast Episodes in 2023, here is number 9
Navigating canine anaphylaxis
Identifying culprits of this allergic reaction to help veterinary teams save their patients’ lives

Podcast CE: Canine cardiology: the practical guide to the mitral valve patient
Learn about the prevalence of myxomatous mitral valve disease, guidelines for staging heart disease, proactive diagnostic workup, the importance of spironolactone and aldosterone blocking, and the benefits of combination therapy for improved outcomes in canine patients

When to take this cough to heart
Dogs with heart murmurs may experience coughing episodes, but dogs that cough may have heart murmurs. Despite a murmur, a dog’s cough might not be cardiac in origin. Then again, it could be. Differentiating the 2 is critical.

Effect of non-traditional vs traditional diets on Irish wolfhounds' heart health
Findings reveal Irish wolfhounds eating nontraditional diets had significantly more VPCs compared to dogs eating traditional diets
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

ECG: wandering pacemaker
ISSN 2398-2942
Subscribe To View
This article is available to subscribers. try a free trial today or contact us for more information., related articles, ecg: principles of interpretation, heart: atrial premature complexes, heart: dysrhythmia, related images, ecg: sinus arrhythmia, ecg: sinus rhythm, register now to access this article free of charge, receive one free article when you register..
- Hidden Free Article URL --> -->
- First Name *
- Last Name *
- Telephone Number: *
- I am a: * Please Select Student Nurse/Technician Veterinarian Vet School Faculty Pet Owner Other
- Country Select Afghanistan Åland Islands Albania Algeria American Samoa Andorra Angola Anguilla Antarctica Antigua & Barbuda Argentina Armenia Aruba Ascension Island Australia Austria Azerbaijan Bahamas Bahrain Bangladesh Barbados Belarus Belgium Belize Benin Bermuda Bhutan Bolivia Bosnia & Herzegovina Botswana Bouvet Island Brazil British Indian Ocean Territory British Virgin Islands Brunei Bulgaria Burkina Faso Burundi Cambodia Cameroon Canada Canary Islands Cape Verde Caribbean Netherlands Cayman Islands Central African Republic Ceuta & Melilla Chad Chile China Christmas Island Clipperton Island Cocos (Keeling) Islands Colombia Comoros Congo - Brazzaville Congo - Kinshasa Cook Islands Costa Rica Croatia Cuba Curaçao Cyprus Czechia Côte d’Ivoire Denmark Diego Garcia Djibouti Dominica Dominican Republic Ecuador Egypt El Salvador Equatorial Guinea Eritrea Estonia Eswatini Ethiopia Falkland Islands (Islas Malvinas) Faroe Islands Fiji Finland France French Guiana French Polynesia French Southern Territories Gabon Gambia Georgia Germany Ghana Gibraltar Greece Greenland Grenada Guadeloupe Guam Guatemala Guernsey Guinea Guinea-Bissau Guyana Haiti Heard & McDonald Islands Honduras Hong Kong Hungary Iceland India Indonesia Iran Iraq Ireland Isle of Man Israel Italy Jamaica Japan Jersey Jordan Kazakhstan Kenya Kiribati Kosovo Kuwait Kyrgyzstan Laos Latvia Lebanon Lesotho Liberia Libya Liechtenstein Lithuania Luxembourg Macao Madagascar Malawi Malaysia Maldives Mali Malta Marshall Islands Martinique Mauritania Mauritius Mayotte Mexico Micronesia Moldova Monaco Mongolia Montenegro Montserrat Morocco Mozambique Myanmar (Burma) Namibia Nauru Nepal Netherlands New Caledonia New Zealand Nicaragua Niger Nigeria Niue Norfolk Island North Korea North Macedonia Northern Mariana Islands Norway Oman Pakistan Palau Palestine Panama Papua New Guinea Paraguay Peru Philippines Pitcairn Islands Poland Portugal Puerto Rico Qatar Romania Russia Rwanda Réunion Samoa San Marino Saudi Arabia Senegal Serbia Seychelles Sierra Leone Singapore Sint Maarten Slovakia Slovenia Solomon Islands Somalia South Africa South Georgia & South Sandwich Islands South Korea South Sudan Spain Sri Lanka St. Barthélemy St. Helena St. Kitts & Nevis St. Lucia St. Martin St. Pierre & Miquelon St. Vincent & Grenadines Sudan Suriname Svalbard & Jan Mayen Sweden Switzerland Syria São Tomé & Príncipe Taiwan Tajikistan Tanzania Thailand Timor-Leste Togo Tokelau Tonga Trinidad & Tobago Tristan da Cunha Tunisia Turkey Turkmenistan Turks & Caicos Islands Tuvalu U.S. Outlying Islands U.S. Virgin Islands Uganda Ukraine United Arab Emirates United Kingdom United States Uruguay Uzbekistan Vanuatu Vatican City Venezuela Vietnam Wallis & Futuna Western Sahara Yemen Zambia Zimbabwe
We hope your free content was what you wanted
For continued access, sign up today and get:.
Instant access to the knowledge of 1,100+ leading veterinarians
Over 28,000 per reviewed articles, images, videos, sounds, diagnostic trees
More than 1,100 client factsheets
On the veterinary diagnosis and treatment of your choice of species: dogs, cats, rabbits, exotics, horses and cattle.
Starting at:
Interested in more great content?
Sign up today..
On the veterinary diagnosis and treatment of your choice of species: dogs, cats, rabbits, exotics, horses and cattle
How can we help you today?
Stray 4-month-old puppy found wandering along highway in Roslindale
BOSTON (WHDH) - A stray 4-month-old puppy was spotted wandering along Cummins Highway Roslindale by a passerby early Wednesday morning, according to the Animal Rescue League of Boston.
The puppy, now named Petunia, was taken to an emergency veterinary hospital and treated for severe demodectic mange, a skin condition that causes fur loss, itchiness, and discomfort, the rescue league said in a statement. It can also affect a dog’s immune system, putting it at risk of serious illness.
“It was clear she was suffering and needed immediate medical attention,” the rescue league said.
After Petunia’s treatment, the city’s animal control division took her to the Animal Rescue League’s Boston Animal Care and Adoption Center, where she will continue to recuperate, the statement said. She is not currently available for adoption, according to the ARL.
“While it is unknown where she came from or how she found her way to the busy roadway, ARL’s focus right now is getting this helpless puppy on the path to recovery,” the rescue league said.
The public can donate to aid Petunia’s recovery on the ARL’s website .
(Copyright (c) 2024 Sunbeam Television. All Rights Reserved. This material may not be published, broadcast, rewritten, or redistributed.)
- 7WEATHER: Cool Pattern, Limited Rain
- 3 people appear in court after arrest in connection with deadly Leominster stabbing
- Counterfeit Patriots Super Bowl ring among items seized in New York
- WATCH: Karen Read trial recap, day 9
Transylvania University offering unique class for dogs and their owners
LEXINGTON, Ky. (WKYT) - Students at Transylvania University are being offered a unique class this May term.
The learners just happen to be man’s best friend, dogs!
“I’m teaching a class called ‘Fido on the Couch’, and in this class, we are working with dogs on training simple behaviors and working on problem behaviors,” said Ellen Furlong, Asistant Professor of Psychology and Neuroscience.
Furlong teaches this May term class for the dogs and students alike.
These K-9 college students are learning basic skills.
Some students bring their pets from home, but for those who don’t have a furry friend, Lexington Pit Crew, a local pit bull rescue, provides a solution.
“We are mostly working with foster dogs. All of our dogs who are here today except for Nellie and Winston, whose owners are in the class today, are up for adoption,” said Furlong.
While much of the class is spent having fun with the dogs, these pups are making contributions to research as they roll around.
“The students are working on developing a foster manual for the Lexington Pit Crew that will include a lot of the things that they’ve learned here so they can share it with the people who are doing the foster work day to day,” said Furlong.
The students are teaching the dogs, and the dogs are teaching them how to show others that all dogs deserve a second chance.
Copyright 2024 WKYT. All rights reserved.

Ahead of Mother’s Day, Kentucky couple gets unexpected arrival

Longtime Lexington radio host dies

KSP looking for missing teens; warn public not to approach them

Wawa set to break ground on Kentucky locations

Lexington nonprofit gets backlash for lack of diversity after display announcement
Latest news.

Large police presence in downtown Lexington

Lexington woman graduates from college more than 50 years after first enrolling

Animal cruelty case under investigation in Lexington; how you can help

London mayor sues woman for defamation
Books | The Book Club: “Wandering Stars” and more short…
Share this:.
- Click to share on Facebook (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Twitter (Opens in new window)
- Food & Drink
- TV Listings
Things To Do
Books | the book club: “wandering stars” and more short reviews from readers, two books earn 3 1/2 stars out of 4.

“Wandering Stars,” by Tommy Orange (Alfred A. Knopf, 2024)

This sequel to Orange’s earlier novel, “There There” (a finalist for the Pulitzer Prize in 2019), “Wandering Stars” is divided into 2 sections (“Before” and “Aftermath”). This division is not only chronological but also stylistic. “Before” opens with the Sand Creek Massacre in 1864 and relates the history of a single Native American family into the 20th century. The characters’ individual experiences of displacement and cultural repression also represent Native American history writ large. Orange employs a sort of linguistic shape-shifting in this section, with individual words of multiple meanings repeated over and over. And I had to read some passages over and over to appreciate the subtlety of his intent. “Before” presents a somewhat mystical space, guiding the reader to feel how earlier Native American generations were so differently connected to their world and spiritual beliefs than their European conquerors.
In contrast, “Aftermath,” set in present-day Oakland, Calif., uses a lot of dialogue to move the story more crisply forward. Several of the characters in “Aftermath” wrestle with what it means to be Native American and their longing for the lost stories and traditional ways of past generations intensifies over successive generations. But the sad through line connecting the 2 sections is substance abuse and addiction, starting with alcohol, peyote and laudanum in the 19th century and landing with heroin and opioids in contemporary Oakland. How the characters cope throughout this novel is uniquely individual, but simultaneously universal. — 3 1/2 stars out of 4; Kathleen Lance, Denver
“Fourteen Days,” by Margaret Atwood, et. al. (Harper, 2024)

During the COVID lockdown, New Yorkers sharing an apartment building gather nightly on the roof to share stories. Written by 36 different renowned authors — including Margaret Atwood, Celeste Ng, Tommy Orange and John Grisham — this book of tales is unified by careful scaffolding into a surprisingly cohesive novel. The stories told are compelling, diverse, and varied in genre — and puzzling out who wrote what (not revealed until the end) makes for intriguing reading. — 3 1/2 stars (out of 4); Neva Gronert, Parker
“Wool,” by Hugh Howey (Broad Reach Publishing, 2012)
The author self-published a post-apocalyptic novel and made history, at least among struggling writers, by becoming a best-seller. He completed four novellas in the series, then obtained a publishing contract. The action of this compelling sci-fi thriller takes place in a silo, a 144-floor underground community of humans. The air above ground is toxic after an unknown catastrophe. Those who attempt to go out and others being punished for misdeeds are “cleansed.” Juliette, a new mayor, attempts to rectify the many social and personal problems of the society but seems doomed to failure. Her battle to survive herself, as well as save the remnants of civilization, leads readers to question the value of our way of life. — 3 stars (out of 4); Bonnie McCune, Denver, bonniemccune.com

“The Hunter,” by Tana French (Viking, 2024)
If you enjoyed “The Searcher,” you will welcome dropping back into our hero Cal Hooper’s life as a retired ex-cop, ex-pat now living a quiet life in a small Irish village. Unexpected events put a quick end to the quietness of that life. A well-paced, compelling mystery with lots of local color and a touch of humor. — 3 stars (out of 4); Kathleen Lance, Denver
Subscribe to our weekly newsletter, In The Know, to get entertainment news sent straight to your inbox.
- Report an Error
- Submit a News Tip
More in Books

Books | New Sara Paretsky novel and more mysteries to solve this month

Books | The Book Club: “The Berry Pickers” and more short reviews from readers

Colorado News | Readers Take Denver cancels 2025 conference after attendees decry “Fyre Festival of books”

Books | The Book Club: “Absolution” and more short reviews from readers

IMAGES
VIDEO
COMMENTS
Often, there is an accompanying change in P wave configuration (wandering pacemaker) with the P waves becoming taller and spiked during inspiration and flatter in expiration. Marked sinus arrhythmia occurs in some animals with chronic pulmonary disease. ... It is commonly seen in dogs, but not often in the clinical setting in normal cats ...
"Dogs might benefit from a pacemaker if they have an arrhythmia (abnormal heart rhythm) or a heart rate that is too slow to support the dog in daily activities," says Dr. Fries. "Some arrhythmias can stop the heart and be life-threatening. Other heart conditions may simply impede the dog's ability to exercise and live a normal life."
The sinus node initiates depolarization of the rest of the heart in a healthy animal, sets the normal rate and rhythm, and is called the normal pacemaker of the heart. It functions as the pacemaker because it is automatic (depolarizes on its own) and does so at a rate faster than the other automatic sites in the heart (AV node and Purkinje fibers).
FIGURE 1. ECG showing a normal sinus rhythm. FIGURE 2. ECG showing a sinus arrhythmia with a regularly irregular rhythm, in which the heart rate increases and decreases in a pattern. This is considered normal in relaxed dogs. FIGURE 3. ECG and illustrations showing a wandering pacemaker.
• A wandering pacemaker (a change in the morphology of the P wave due to a change in pacemaker location or conduction) ... • Sinus arrhythmia is a normal rhythm variation in the resting dog, often correlated with varying levels of sinus node vagal tone, which changes with respiration ...
The implantation of a pacemaker is today a common procedure in dogs. Veterinary cardiologists who are experienced in the implantation of pacemakers and the programming of these pacemakers can best insure the best treatment for afflicted dogs. The response to treatment is usually very good. In dogs that also have the tachycardia this is treated ...
A wandering pacemaker means that the P waves vary in height and may even be negative temporarily (Figure 4-26). Sinus arrest is defined as a prolongation of the P-R interval longer than twice the usual P-P interval. ... The QRS complexes are usually narrow (60 msec in dogs; 40 msec in cats) unless a bundle branch block is present. ...
Pacemakers are a life-saving intervention. These electronic devices can also help improve the quality of life in dogs diagnosed with certain heart problems. An artificial pacemaker is implanted to maintain the heart rate by stimulating the contraction of the heart muscles. A pacemaker implant can help ensure that your dog has a normal heart rate.
When the P wave varies in association with respiration (RR interval), it is referred to as a "wandering pacemaker." The P wave also may vary within a dog during serial recordings. The P-wave amplitude can change quickly with changes in heart rate. The faster the heart rate, the taller the P wave. The T wave also changes with heart rate.
From these early studies the discovery of widespread initiation of atrial depolarization that seemed to originate outside the limits of the central sinus node helped to explain the wandering pacemaker that is common in dogs with sinus arrhythmia (Boineau et al., 1980: Schuessler et al., 1986). At the time of these investigations mapping of the ...
Abstract. the implantation of pacemakers is becoming a more widely available therapy in dogs, with approximately 50 such procedures performed annually in the uk. this article provides the general practitioner with an overview of pacemakers and their indications in veterinary practice, emphasising the clinical conditions that require pacemaker ...
A pacemaker is a device that, through surgical intervention, is integrated into the dog's heart to help it maintain a beating frequency appropriate to its needs. It consists of a pulse generator (battery), an electrode that connects it to the heart's myocardium, and programming equipment. Its use is better known in humans, but as we indicated ...
Pacemaker and lead implantation procedures have been performed within the veterinary field for over 40 years, with the first implant in 1967 via a thoracotomy and epicardial placement of 2 leads for fixed rate pacing of 70 bpm [].Most veterinary patients undergoing pacemaker implantation today receive transvenous lead implants, resulting in a minimally-invasive procedure [2,3,4, 9, 14].
Introduction. The sinoatrial node (SAN), described as the primary cardiac pacemaker, is the source of intrinsic electrical activation consistently driving the coordinated rhythmic contractions of the mammalian heart [1,2].It initiates the heartbeat via a combination of pacemaker cells which generate spontaneous cellular electrical signals, and specialized conduction pathways, which conduct the ...
Cardiac anesthesia and surgery can generally be described as high risk, high reward. Pacemaker implantation exemplifies this since no other group of patients has such a high risk of sudden asystole and death, but yet can have a dramatic improvement in quality of life with successful implantation. Pacemakers implanted in dogs most often employ a ...
Pacemaker-lead-associated thrombosis was identified in 27/260 (10.4%) dogs with echocardiographic follow-up. The median time from pacemaker implantation to PLAT diagnosis was 175 days (6-1853 days). Echocardiography identified the thrombus on the lead in 26/27 (96.3%) dogs, whereas computed tomography was required to identify the thrombus in ...
An atrial premature complex (APC) is an electrocardiographic (ECG) abnormality. Cause: indicates an extra heart beat arising from a focus of electrical activity in the atria other than the sinoatrial node, which is the primary cardiac pacemaker. Signs: APCs do not usually cause clinical signs but condition may worsen and therefore should be ...
Dogs with respiratory disease can show pronounced sinus arrhythmia with wandering pacemaker; the short cycles can resemble premature atrial complexes. Management of sinus rhythm disturbances is focused first on treating any underlying conditions. Occasionally inappropriate sinus tachycardia is treated with a beta-blocker.
Native If in nonexpressed atrial myocytes was 7±4 pA at −130 mV (n=5), whereas HCN2+GFP LA had expressed pacemaker current ( IHCN2) of 3823±713 pA at −125 mV (n=10) and 768±365 pA at −85 mV. Conclusions— HCN2 overexpression provides an If -based pacemaker sufficient to drive the heart when injected into a localized region of atrium ...
View ECG: wandering pacemaker & more Canis resources at Vetlexicon. Over 28,000 peer-reviewed resources: Bovis, Equis, Felis, Lapis & Exotis. ... On the veterinary diagnosis and treatment of your choice of species: dogs, cats, rabbits, exotics, horses and cattle. Starting at: £40 /month. View Pricing Options Extend My Trial.
Introduction. The use of artificial cardiac pacemakers (APs) to treat symptomatic bradyarrhythmias in veterinary medicine is commonplace. Third-degree atrioventricular block (3AVB) is the most frequently encountered bradyarrhythmia prompting pacemaker implantation in the dog [1], [2], [3].The underlying lesion leading to 3AVB in dogs is most often believed to be non-specific fibrosis or ...
WHDH TV 7NEWS WLVI TV CW56 Sunbeam Television Corp 7 Bulfinch Place Boston, MA 02114 News Tips: (800) 280-TIPS Tell Hank: (855) 247-HANK
These K-9 college students are learning basic skills. Some students bring their pets from home, but for those who don't have a furry friend, Lexington Pit Crew, a local pit bull rescue, provides ...
"Wandering Stars," by Tommy Orange (Alfred A. Knopf, 2024) "Wandering Stars," by Tommy Orange (Alfred A. Knopf, 2024) ... Dogs saves owner from attempted sexual assault in Fort Collins park.