- Short report
- Open access
- Published: 09 December 2005

TRIPS, the Doha Declaration and increasing access to medicines: policy options for Ghana
- JC Cohen 1 ,
- M Gyansa-Lutterodt 2 ,
- K Torpey 3 ,
- LC Esmail 4 &
- G Kurokawa 5
Globalization and Health volume 1 , Article number: 17 ( 2005 ) Cite this article
24k Accesses
13 Citations
3 Altmetric
Metrics details
There are acute disparities in pharmaceutical access between developing and industrialized countries. Developing countries make up approximately 80% of the world's population but only represent approximately 20% of global pharmaceutical consumption. Among the many barriers to drug access are the potential consequences of the Trade Related Aspects of Intellectual Property Rights (TRIPS) Agreement. Many developing countries have recently modified their patent laws to conform to the TRIPS standards, given the 2005 deadline for developing countries. Safeguards to protect public health have been incorporated into the TRIPS Agreement; however, in practice governments may be reluctant to exercise such rights given concern about the international trade and political ramifications. The Doha Declaration and the recent Decision on the Implementation of Paragraph 6 of the Doha Declaration on the TRIPS Agreement and Public Health may provide more freedom for developing countries in using these safeguards. This paper focuses on Ghana, a developing country that recently changed its patent laws to conform to TRIPS standards. We examine Ghana's patent law changes in the context of the Doha Declaration and assess their meaning for access to drugs of its population. We discuss new and existing barriers, as well as possible solutions, to provide policy-makers with lessons learned from the Ghanaian experience.
Introduction
The disparity in pharmaceutical access between developed and developing countries is stark. Developing countries make up approximately 80% of the world's population but only represent approximately 20% of global pharmaceutical consumption[ 1 ]. Market failures, government failures and income differences account for this persisting inequity[ 2 ]. Specifically, high drug costs, weak or corrupt institutions, contributing to less than effective pharmaceutical purchasing and distribution systems, and the potential consequences of the Trade Related Aspects of Intellectual Property (TRIPS) Agreement all constrain drug access.
Many developing countries have recently modified their patent laws to conform to TRIPS standards, raising the urgency of TRIPS' potentially detrimental impact on drug supply and access. However, recent developments such as the Decision on the Implementation of Paragraph 6 of the Doha Declaration on the TRIPS Agreement and Public Health may offer developing countries more freedom to use TRIPS safeguards to address public health needs.
This article focuses on Ghana, a developing country that recently changed its patent laws to conform to TRIPS standards. While Ghana has made strides in improving public health, the country has urgent and serious health needs that cannot be met by the existing system. Improving pharmaceutical access is one of the core challenges facing the Government. As such, there is a menu of choices available for possible use. These include "Paragraph 6", compulsory licensing, parallel importing and attracting investment for the local production of essential medicines to combat HIV/AIDS, malaria and tuberculosis. In this paper, we discuss a selection of pharmaceutical policy choices available to governments that may lead to improved access to medicines. We do this specifically through the case of Ghana. As a test case, Ghana represents the dilemma faced by many developing countries -"make or buy." That is to say, should a government invest more in local production or continue to import medicines? In short, we examine Ghana's patent law changes in the context of the Doha Declaration and assess their meaning for access to drugs of its population. New and existing barriers will be discussed and options for addressing them proposed, to provide policy-makers with lessons learned from the Ghanaian experience.
This paper is based on research conducted for the UK Department for International Development (DfID)[ 3 ]. Our study implicitly focuses on the basic pharmaceutical public policy issues that currently face decision-makers in Ghana: 1) how to improve pharmaceutical coverage for the majority of Ghanaians and most importantly for the poor; 2) how to make treatment and drugs more affordable; and 3) how to ensure the government gets maximum value from its pharmaceutical budget. We collected data through: a review of "hard" documents (recent academic literature, policy documents, recent country studies, laws and regulations relating to drug access issues in Ghana and internationally), interviews with key stakeholders and informants to identify major gaps in drug access in Ghana, and inputs from the Access to Medicines Ghana Initiative Advisory Group.
We organize our paper as follows: first, we describe Ghana and its pharmaceutical system; second, we discuss the TRIPS Agreement and the Doha Declaration; third, we discuss Ghana's 1992 Patent Law and 2003 Patent Act; fourth, we discuss Ghana's new Patent Act in the context of the Doha Declaration and of pharmaceutical access in Ghana, followed by conclusions.
The State of Pharmaceuticals in Ghana
Ghana has made significant improvements in its overall health status over the past few decades, with life expectancy reaching 57 years in 2002 and infant mortality declining to 56 per 1000 live births. Despite these improvements, there are significant health issues facing the country: approximately 3.6% of the population is infected with HIV/AIDS, malaria accounts for 40% of outpatient visits and 25% of mortality under the age of five and the annual risk of tuberculosis infection is approximately 1–2%[ 4 ]. High mortality rates, frequent epidemics, unequal access to health services, and uneven health outcomes throughout the country are also major problems.
Pharmaceuticals are available in many health facilities across the country; however, access is largely limited due to financial barriers for most of the population, particularly the poor. According to the World Bank, Ghana had a per capita income of US$380 (2004), which is about one-fifth below the average of US$490 for sub-Saharan Africa. Moreover, a recent study indicates that 40 percent of Ghana's population earns less than minimum wage with this proportion increasing in rural areas[ 5 ]. As a result, the poverty level makes it difficult for patients to purchase drugs. For example, HIV/AIDS patients receiving one-month of anti-retroviral therapy paid for by the Global Fund are still required to pay 10% of the costs of medicines, at approximately 50,000 cedis (over $US 5). In real terms, it would require a person who is earning the minimum wage more than 5 working days to cover the co-payment. In a small random sample of interviews with patients at the HIV/AIDS Clinic in St. Martin's Hospital, Agomanya, we observed that many patients were not working at all and had to borrow money from family members to cover this co-payment.
Until recently, Ghana's public health and pharmaceutical system operated under the "cash & carry" (C&C) model, which assumes that drug co-payments can help finance and, therefore, improve the delivery of primary health care services. This system involved a series of self-financing revolving drug funds (RDF), which cascaded down each institutional level, marking up the basic purchase price for drug products to obtain revenue to re-supply the products. Additively, these mark-ups could also increase the price of a drug well beyond the reach of most Ghanaians. The government provided exemptions for co-payments for specific categories, including TB patients, psychiatric patients, children under five years of age, the indigent, pregnant women and the elderly. Identifying who is truly indigent is difficult to do in Ghana because poverty is viewed from a socio-cultural point of view as "shameful" and many poor people are reluctant to admit it. Essentially, this system did not achieve its intent to provide widespread affordable access to medicines for the population.
The Government of Ghana declared its intention to abolish the C&C system and passed a National Health Insurance Bill in 2003, which recently has been implemented. Several districts have also introduced health insurance in their localities. These insurance measures may serve to improve access to medicines; however, their effectiveness is yet to be observed. Importantly, the draft minimum package for pharmaceuticals includes medications for outpatient and inpatient services. Antiretrovirals are covered under different arrangements using the Global Fund to fight AIDS, Tuberculosis and Malaria. The Ministry of Health is reviewing exemption policies and the proposed National Health Insurance drug list to ensure consistency with the Essential Drug List. Despite the difficulties noted above, the Insurance system hopes to use local structures to identify who is "poor" to ensure these categories can access care. A national pricing policy, informed by a comprehensive examination of pharmaceutical pricing models internationally, can also facilitate better financial access of the population to medicines.
Pharmaceutical mark-ups are another policy issue that needs reform. The international research-based and generic pharmaceutical industries provide discounted medicines to Ghana, however once products arrive in Ghana, mark-ups between 11% to 275% wipe out many price advantages[ 3 ]. Tax and tariff rates vary but are applicable to all medicines, except public sector procurement done according to the Essential Drug List. In the private sector, depending on the local agent or manufacturer, the cost of antiretrovirals can exceed 32.5% more than the discounted price obtained through the Accelerated Access Initiative (AAI). In some cases, the private health facility adds further margins to increase the cost. In order to effectively address mark-ups, national tax, tariff and mark-up policies need to be reviewed to determine what policy changes could facilitate more affordable prices. Government can regulate wholesale and retail mark-ups on essential medicines. Policies regarding the private sector management chain and public sector supply management chain need examination and adjustment to make medicines more affordable for patients.
Ghana has potential to supply more of its medicine needs; local manufacturing accounts for 20% of the pharmaceutical market share. There are about 30 pharmaceutical manufacturing facilities in Ghana and about 17–18 produce all year round. Most raw materials needed for local manufacture are imported and subject to duties, taxes and tariffs, which erode the potential cost advantage that local manufacturing can provide. Currently, manufacturers pay 12.5% VAT (Value Added Tax), 0.5% EDIF (Economic Development Investment Fund), 0.5% ECOWAS levy, handling and inspection charges, GCNet charges (0.004% of cost and freight)[ 3 ]. However, Schedule 1A of the 34 materials of Active Ingredients of Essential Medicines are exempt from tax. Manufacturers apply a mark up that can range from 10 to 40%. Wholesalers add a further 10 to 20% when selling to the retailer. Then, the retailer adds another margin of 20 to 50%. All of these margins obviously increase the price of the drug for the patient, thereby contributing to the financial barrier to medicines. To help local industry, the government restricts the importation of 17 pharmaceutical products (e.g. paracetamol, chloroquine). Local production is beneficial given that it also provides employment for the population; however the barrier of limited human and technological capacity must first be overcome.
The TRIPS Agreement and the Doha Declaration
By way of brief background, the TRIPS Agreement provides minimum standards for intellectual property law and procedures and remedies that should be available so rights holders can enforce their rights effectively[ 6 ]. The default principle concerning patents is that they should be available for any invention, whether product or process, in all fields of technology without discrimination[ 6 ]. With respect to pharmaceutical patents, the minimum TRIPS obligations include 20 years of patent protection from the inventor's filing date (Article 33), patent rights free of discrimination against the origin of invention or production (Article 27.1), and exclusive marketing rights for the entire patent duration (Article 28)[ 7 ]. Transitional periods are granted before TRIPS requirements for patent protection must be met; the deadline for least-developing country members was ultimately extended to 2016 (Articles 65 and 66)[ 6 ].
The TRIPS Agreement also outlines provisions around patent rights for member states. For example, Articles 8.2, 31(k) and 40 offer flexibility to member countries to prevent or remedy anti-competitive practice[ 8 ]. Article 30 facilitates an early working provision, allowing the limited use of an invention without the patent holder's authorization[ 9 ]. Generic companies can use this provision to obtain product approval, facilitating immediate entry into the market after patent expiration. Article 31 permits a government to issue a compulsory license to a third party without the patent holder's consent, if justified in the public interest[ 7 ]. Compulsory licensing allows governments to pursue the local production of medicines as one strategy to improve access of the population to essential medicines. Parallel importing, legally pursuant to TRIPS Article 8.1 and Article 6, is the import and resale in a state without the consent of a patent holder, of a patented product in another market. Its rationale is to allow governments and others to "price shop" internationally for pharmaceutical products, based on the underlying principle that the patent holder has been rewarded through the first sale and thus has "exhausted" rights. Compulsory licensing and parallel importation are the focus of this paper.
In practice, governments may realistically be reluctant to exercise TRIPS provisions given some concern about political and economic ramifications, particularly in the area of trade sanctions. The Doha Declaration, issued by the WTO in November 2001, partially aimed to address this concern. It states " [the] TRIPS Agreement does not and should not prevent members from taking measures to protect public health... [and it] should be interpreted and implemented in a manner supportive of WTO members' right to protect public health and, in particular, to promote access to medicines for all" [emphasis added][ 10 ]. Furthermore, the Declaration specifically reaffirms member countries' rights to determine the grounds on which compulsory licenses may be issued, to determine what constitutes a national emergency or circumstance of extreme urgency, and to determine their own regime for the exhaustion of intellectual property rights.
The WTO has, however, not explicitly defined the legal status of the Declaration. An unofficial explanation of the Declaration, available on the WTO website, states that the Declaration provides "important guidance" to individual members and WTO dispute settlement bodies in the interpretation of TRIPS[ 11 ]. Correa identifies the Declaration as a "strong political statement" and "a 'subsequent agreement' between the parties regarding the interpretation of a treaty or the application of its provisions, under Article 31.3(a) of the Vienna Convention on the Law of the Treaties"[ 12 ]. Ultimately, the functional application of the Declaration is to interpret TRIPS[ 13 ], however as Reichman and Hasenzahl note[ 14 ], the exact legal status of the Declaration will not be clear until its practical application has been observed through future WTO panels and the Appellate Body.
The Declaration was partially an effort to interpret Article 31(f) of the TRIPS Agreement, which states that compulsory licensing shall be "predominantly for the supply of the domestic market." Given that the majority of developing countries lack the domestic capacity or technical expertise to manufacture on-patent pharmaceuticals, the interpretation of this terminology is crucial for ensuring access to medicine for the poor in many developing countries. On August 30, 2003, the WTO issued a temporary solution, the "Decision on the Implementation of Paragraph 6 of the Doha Declaration on TRIPS Agreement and Public Health"[ 15 ]. The Decision temporarily waives Article 31(f), permitting countries with local manufacturing capacity to issue compulsory licenses to produce and export drugs to countries without adequate manufacturing capacity, in return for a pledge from countries not to use the Decision "...to pursue industrial or commercial policy objectives"[ 16 ]. Eligible importing countries include least-developed countries or a country that can demonstrate insufficient or no manufacturing capacity for the purpose of meeting its needs. No country, at the time of writing, has yet notified the WTO of its intention to operate as an importer under this decision. Countries may in fact be reluctant to do so as a result of economic and political pressure.
Ghana's Patent Law
The Patent Law of 1992 provided for the protection of patents in Ghana until recently. This law provided that all inventions, products or processes which were "new, involve an inventive step and are industrially applicable," were patentable (Sec.2)[ 17 ]. Pharmaceuticals were considered patentable inventions and patent duration was 10 years (Sec.31(1)). The law permitted compulsory licensing in cases of no or insufficient local working of the patented invention (Sec.45(1)), based on the interdependence of patents (Sec.46), and for products or processes declared to be of vital importance to defence, economic or public health interests (Sec.47). Section 30 considered the patent holder's right exhausted only when he put his patented product on the Ghanaian market, rendering parallel importation impossible. To meet all TRIPS obligations and take advantage of its safeguards, the Ghanaian government reviewed the Patent Law of 1992 and passed a bill to replace it in early 2003.
The changes introduced in the 2003 Patent Act removed some legal tools that may have helped improve access to medicines[ 18 ]. Under Section 7 of the 1992 Patent Law, the Ghanaian government had the authority to temporarily exclude inventions or discoveries, such as pharmaceuticals, from patentability "... in the interests of national security, economy, health or any other national concern." The 2003 Patent Act removed this exception. Arguably, the government of Ghana could have excluded specific pharmaceutical products from patentability as a temporary means to address urgent public health concerns. Temporary excludability is particularly useful when procedural requirements to compulsory licensing cannot be met[ 9 ]. However, as Correa explains, a literal interpretation of Article 27.1 does not allow the exclusion of pharmaceuticals[ 9 ]. He notes that under TRIPS Article 27.2 ordre public and Article 8.1 "...pharmaceuticals might conceivably be excluded from patentability, but neither appear sufficient to justify this exclusion except in limited circumstances,"[ 9 ]. In any case, the option of using temporary excludability appears unviable at the present time.
Section 13(2) addresses the royalty rate for compulsory licenses: "... [t]he exploitation of the invention...shall be subject to the payment to the owner of an adequate remuneration, taking into account the economic value of the Minister's decision as determined in the decision..."[ 18 ]. Adequate remuneration is undefined, allowing for the negotiation of prices, which could have either positive or negative effects on price control and access to medicines. The Ghanaian government has since developed administrative guidelines, proposing the creation of a committee that would determine the level of compensation to be given to a patent holder. It proposed that remuneration for drugs used to treat HIV/AIDS, TB and malaria not exceed 1% of the retail price. In Canada, a country with extensive history of compulsory licensing, a royalty rate of 4% was used[ 19 ]. Furthermore, Canada's recent Jean Chrétien Pledge to Africa Act may offer useful guidance, as the royalty rate varies from 4 to 0.02% depending upon the importing country's standing on the UN Human Development Index[ 20 ].
Section 12(1) of the 2003 Patent Act incorporated Article 33 of TRIPS, doubling the period of patent protection to 20 years. The result will be a delay in the entry of generic competition, and since generic competition tends to lower drug prices, a reduction in overall cost-savings is likely[ 21 ]. This provision does not offer any flexibility to member countries; therefore the ensuing barriers will require circumvention via other TRIPS safeguards or policy alternatives. As noted earlier, the Ghana's major disease burden includes malaria, TB and HIV/AIDS. While these diseases can be treated with off-patent medications, extended patent life will be problematic in situations where no other therapeutic options are available[ 22 ]. Specifically, evidence of resistance to traditional antimalarial therapy ( e.g ., chloroquine) exists and patients who develop resistance to anti-retroviral medications or experience treatment failure will need access to new, patented medicines in the future. The increase in duration of patent protection impedes Ghana's autonomy over defining their population's therapeutic needs.
Changes were also introduced in the 2003 Patent Act that may promote access to medicines. Section 11(4a) of the 2003 Patent Act allows the international exhaustion of intellectual property rights. This legalizes the parallel importation of lower-priced pharmaceuticals from other countries into Ghana, which will be discussed in detail below. Compulsory licenses can now be issued in circumstances of anti-competitive practice, which allows Ghana to remedy abusive practices and excessive prices, potentially increasing the availability of affordable medicines[ 9 ]. Potential also exists to use TRIPS' anti-competitive provisions, accompanied by appropriate national competition policy, to promote the development of the local pharmaceutical industry[ 8 ]. The successful suit by the Aids Law Project (ALP) against two major pharmaceutical companies with the South African Competition Commission in 2002 illustrates this potential. Other positive changes include new procedures in granting compulsory licenses: a waiver to seek a voluntary license in "cases of national emergency or other circumstances of extreme urgency," (Section 13(10)) and ministerial authorization instead of the previous lengthy and resource-intensive requirement of legislative authorization (Sec.13(1)).
The 2003 Patent Act widens the provision for issuing a non-voluntary license under local working requirements. Local working requires the manufacturing of a patented product to a minimum degree within the country, potentially stimulating growth of the local pharmaceutical industry. Specifically, the 2003 Patent Act allows non-voluntary licenses in situations where "...the patented invention is not exploited or is insufficiently exploited by working the invention locally, or by importation in the country," (Sec.14(1)) [ 18 ]. Whereas the 1992 Patent Act listed four relatively specific instances, where the compulsory license could be invoked if the invention was not being worked, the 2003 Patent Act makes this more open-ended. The limits of this new clause will likely be drawn by the TRIPS agreement and legislative intent during drafting the Patent Bill in Ghanaian courts; its exact interpretation is still unclear. Given the USTR complaint against Brazil regarding its local working requirement, however, the current political feasibility of including and invoking such a clause is tenuous[ 19 ].
How will these developments impact access to medicines in Ghana?
Compulsory licensing.
Ghana's 2003 Patent Act, TRIPS and the Doha Declaration offer Ghana sufficient legal ground to use compulsory licensing to address its public health concerns. Compulsory licensing can be used for either local pharmaceutical production or importation, however the latter may be more feasible in the short-term. This will be discussed below. Paragraph 5b of the Declaration explicitly reaffirms the right of countries to "...grant compulsory licenses and the freedom to determine the grounds upon which such licenses are granted." As Correa notes, Paragraph 5b merely states the obvious: Article 31 of TRIPS only requires certain conditions for the granting of compulsory licenses, "but it does not limit the grounds on which such licenses can be granted"[ 12 ]. Paragraph 5(c) further facilitates compulsory licensing through the recognition that "public health crises ... can represent a national emergency or other circumstance of extreme urgency" and clarifies that members need not declare a "fully-fledged national emergency"[ 23 ].
From a political perspective, the feasibility of using compulsory licensing to address public health concerns has also become more favourable. One event suggesting this is the abandonment of a pharmaceutical industry lawsuit seeking to remove South Africa's amendment in its Medicines and Related Substances Act, which would permit compulsory licensing and parallel importation. The suit was abandoned due to international pressure and the resolve of the South African government. Other events include Brazil's successful use of the threat of compulsory licensing in negotiations to obtain significant discounts of 40–65% on patented antiretrovirals from Roche and Merck and a public statement by Boehringer-Engelheim in 2003 that it will not interfere in the issuance of compulsory licenses and will respect the Doha Declaration[ 24 ]. The World Health Organization (WHO) also explicitly supports developing countries in the use of TRIPS safeguards to promote access to medicines[ 25 ]. Given these developments, political and legal repercussions from other powerful countries are less likely. For a country to make effective use of compulsory licensing a number of other potential barriers must be addressed and certain requirements must be met.
Effective implementation of compulsory licensing requires the adequate know-how and administrative infrastructure, however many developing countries, including Ghana, do not have this requisite capacity[ 26 ]. Article 67 of the TRIPS Agreement requires developed countries to provide technical assistance, "on request and on mutually agreed terms and conditions", to developing and least-developed countries to help address such gaps. Developing countries like Ghana should use this provision and approach developed countries and international organizations for support.
The usefulness of compulsory licensing, for local production or as a negotiating tool, largely depends on whether the appropriate technological and production capacity exists and whether appropriate human resources are available. The experience of Brazil provides an illustrative example on this point. Cohen and Lybecker argue that Brazil's success is based on three main factors: first, the threat posed by Brazil is credible in that it has a viable local industry; second, Brazil's market is "...one of the largest in Latin America and among the top ten globally;" third, Brazil initiated its threats with the strong support of the international community[ 24 ]. As we noted earlier, the international community continues to be supportive of developing countries' use of compulsory licensing to address public health needs. In comparison to Brazil, however, Ghana's pharmaceutical market is small which may make the process economically unfeasible. The viability of Ghana's local industry is also questionable as approximately 30 pharmaceutical manufacturing facilities exist but only 17–18 produce throughout the entire year. Careful cost-benefit analysis of the value of domestic production versus the importation of pharmaceuticals is necessary to determine whether Ghana can benefit from compulsory licensing for local production, as it is commonly more economical to import medicines than to produce them locally.
- Parallel Importation
Parallel imports are of particular importance in meeting public health needs since the pharmaceutical industry generally sets differential prices globally for the same medicine. Thus, parallel importation of a patented medicine from a country where it is sold at a lower price will enable more patients in the importing country to gain access to cheaper drugs (whether international exhaustion applies to medicines produced under compulsory licensing, however, is still a live debate; see Abbott 2002). Paragraph 5(d) of the Declaration explicitly reaffirms members' freedom to determine their own regimes for the exhaustion of intellectual property rights without challenge. Ghana's 2003 Patent Act finally facilitates this by incorporating this provision; it allows parallel importation only under the condition that the product to be imported is already "put on the market in any country by the owner of the patent or with the owner's consent," (Sec.11(4a)).
Parallel importing introduces more challenges. Administrative capacity issues exist with parallel importation as with compulsory licensing. In Ghana, import permits for companies engaged in the parallel importation of drugs are difficult to obtain at this time, which may pose another barrier. Parallel importation increases the opportunity for the influx of sub-standard products and thus attendant recall problems. Some critics argue that parallel importing acts as a disincentive to differential pricing by research-based pharmaceutical companies due to a risk of diversion of low-cost products to lucrative, developed country markets; however, as Outterson notes "...empirical evidence to date does not indicate a sizable arbitrage market in ARVs from low income countries into the high income countries"[ 20 ]. Furthermore, there have been no reported cases of diverted drugs from Ghana to other markets. The European Commission's (EC) May 2003 Regulation to facilitate differential pricing may provide another option while lessening some of the industry's re-exportation concerns. The EC regulation provides anti-diversion measures against specific pharmaceutical products and requires manufacturers to reduce their essential medicines export prices to developing countries by "75% off the average 'ex factory' price in OECD countries, or at the cost of production plus 15%"[ 27 ]. If parallel importation is to be useful to Ghana, administrative, institutional and managerial capacity must be developed for effective implementation, to prevent the unlawful importation and exportation of products and to ensure quality control.
Importation Pursuant to Paragraph 6
If Ghana decides against using its' local industry for the production of generic medicines, Paragraph 6 may offer another potential solution. While some critics have viewed this provision favourably, others have criticized it as too administratively complex for developing countries. Correa explains that the implementation of the Paragraph 6 Decision requires a number of steps, among which include: 1) in most cases, compulsory licenses issued by importing and exporting countries, 2) the importing country's establishment of insufficient or no local manufacturing capacity in the specified pharmaceutical sector, 3) importer notification to the WTO of its intention to use the system detailing product(s) requested and quantities (accompanied by confirmation of insufficient manufacturing capacity and that a compulsory license is or will be granted), and 4) notification of the exporting country's compulsory license to the WTO and the conditions attached[ 28 ]. Paragraph 4 of the Decision requires the importing country to "take reasonable measures within their means ... to prevent re-exportation"; this requires countries to implement anti-diversion measures including special marking and labelling of the product(s)[ 15 ]. An obstacle might be introduced with strict data protections laws, as exporters must either obtain authorization from the patent holder to obtain efficacy and toxicity data or when denied, perform its own clinical studies to collect this data; this can increase the exporter's costs, therefore increasing the drug cost in the importing country[ 28 ]. Additional obstacles and delays may depend on the importing country's national laws on product registration and the exporting company's capacity to manufacture the specified product[ 28 ]. Lastly, the Decision limits the exporting country's compulsory license to a "single-supply basis," implying that this entire process must repeat for each new request[ 28 ].
Clearly, administrative barriers may hinder some developing countries from using this; however, Article 67 may be used to obtain external assistance to overcome these gaps. Some critics have also claimed that the Decision will not benefit smaller-sized markets, providing minimal incentive to exporting manufacturers. Paragraph 6 of the Decision may help alleviate this problem. It waives the obligations of TRIPS article 31(f) "to the extent necessary to enable a pharmaceutical product produced or imported under a compulsory license in that member to be exported" to other developing or LDCs that are party to the same regional trade agreement and share the health problem in question. Theoretically, this allows a country like Ghana to harness economies of scale and generate more incentive for generic manufacturers to export. However, such a regional trade area must have been formed in conformity with the provisions of Article XXIV of GATT[ 15 ].
To date, only Canada, Norway and the Netherlands have passed legislation to allow export of pharmaceuticals under this provision[ 29 , 30 ]. To implement Paragraph 6, developing countries will have to address barriers introduced by these exporting countries. For instance, the recently passed Canadian bill contains a restrictive list of medicines that can be produced for the purposes of Paragraph 6. Norway's legislative counterpart is less restrictive. Whether exporting countries will be able to satisfy the global demand for affordable, generic drugs will be observed.
It is also unclear whether a developing country like Ghana can use Paragraph 6 and if so, under what circumstances. LDCs are automatically eligible, but since Ghana is a developing country, it is required to examine its local manufacturing capacity and establish that "...excluding any capacity owned or controlled by the patent owner, it is currently insufficient for the purposes of meeting its needs"[ 15 ]. Ghana has been classified as a country that can reproduce drugs as long as it imports the active ingredients, therefore a cost-benefit analysis is necessary to determine whether or not local manufacture is both technically and economically viable[ 10 ]. Whether the lack of economic viability would be considered as "insufficient local manufacturing capacity" in the event of a dispute is questionable. Correa argues that if low-priced medicines cannot be produced because "...meaningful economies of scale have not been reached..." then one of the main objectives of the Doha Declaration, "to promote access to medicines for all," cannot be reached[ 12 ]. However, reports indicate that the US informed the Philippines and other countries that it does not consider "economic efficiency" as valid ground for the use of Paragraph 6[ 31 , 32 ]. It is also important to note that the General Council Chairperson's Statement on 30 August 2003, includes a mechanism that allows any member to challenge another member's "interpretation and implementation" of the Decision "with a view to taking appropriate action"[ 16 ]. The legal implications of the Chairperson's Statement must still be observed; it is unclear whether a developing country like Ghana will be able to use Paragraph 6 without legal challenge or political and/or economic consequences.
Strengthen local industry capacity
The TRIPS Agreement has several provisions, which deal explicitly with the issue of technology transfer. For instance, article 7 states, "The protection and enforcement of intellectual property rights should contribute to technological innovation and to transfer and disseminate technology , to the mutual advantage of producers and users of technological knowledge and in a manner conducive to social and economic welfare and to a balance of rights and obligations" [emphasis added]. Despite such provisions, little technology transfer to developing countries has taken place[ 33 ]. To strengthen local industry, developing countries like Ghana should still pursue initiatives to absorb new technology. Public-private partnerships (PPPs) may be one mechanism to achieve this. Generally, PPPs require private sector companies to provide the technology and expertise while public sector partners provide development funding and help ensure access to the medications. PPPs can facilitate technology transfer to developing and least-developed countries while creating opportunities to initiate research into developing country diseases[ 23 ].
Pooled Procurement
Pooled procurement is a cost-containment strategy that can assist developing countries in financing of the drug needs of their populations, as it is the one area of the drug supply management cycle that can offer the greatest amount of cost savings. For example, the Eastern Caribbean Drug Service (ECDS) used pooled procurement to lower pharmaceutical expenditure by an average of 44%[ 34 ]. The recently established West Africa Manufacturers Association has put in place mechanisms to take advantage of the economics of scale in the pooled procurement of both raw materials and finished products. In (Economic Community of West African States), political realities have to be addressed given that Francophone West-African countries are already involved in pooled procurement procedures. Thus, if Ghana participates, it would have to comply with established procedures. Ghana must carefully examine the costs and benefits of different procurement policies to determine which ones are most viable and cost-effective. Effective implementation, to be sure, demands institutional capacity, financial management systems, quality assurance and transparency.
Voluntary Differential Pricing Arrangements
Ghana can also pursue the procurement of affordable drugs through voluntary differential pricing arrangements. These arrangements can operate through supplier's charity, desire for favourable public relations, or other criteria not immediately or apparently related to market forces. Currently, limited implementation of differential pricing outside of anti-retroviral therapies occurs in Ghana. As noted earlier, the research-based pharmaceutical industry often cites the risk of re-exportation of these drugs to developed country markets as a barrier to scaling up these initiatives, despite the lack of sufficient evidence. To mitigate these concerns, pharmaceuticals firms often require that recipients of their drugs sign a supply agreement that indicates that the recipient will take measures to ensure the security of the drug supply they receive. To further lessen this risk, companies have special packaging and labelling of drugs provided under special programmes like the Accelerated Access Initiative. Developing countries like Ghana can comply with these measures with assistance from firms and established programs to further encourage differential pricing arrangements.
In this paper, we discuss several possibilities for working within the TRIPS regime to gain better access of the population to medicines. These options include compulsory licensing, parallel importing, technology transfer, local production and voluntary differential pricing. We put forward some favoured policy choices for Ghana. First, we encourage Ghana and its Access to Medicines (ATM) Advisory Committee to consider local production. Local manufacturing can be an effective option if human and technological capacity is scaled up. However, we emphasize that this option should only be pursued if it makes economic sense. As a start, an objective cost-benefit analysis should be done to determine whether it makes economic sense for Ghana to pursue local production. Among the alternatives available to strengthen local industry include more aggressive technology transfer.
Next, we encourage the use of compulsory licensing. If Ghana decides to pursue compulsory licensing, it must then address administrative and knowledge barriers. This can be achieved through obtaining support from developed countries and/or international organizations on the effective implementation of compulsory licensing. There is great potential for Ghana particularly given the Government's commitment to build up its knowledge base in this area. In September 2004, members of the Ghanaian Access to Medicines (ATM) Advisory Committee visited Canada to learn about Canada's past experience with compulsory licensing and what measures could be applied to Ghana. If, however, Ghana determines that it is more technically or economically feasible to pursue importation, Paragraph 6 may provide an option. Ghana will first have to establish insufficient manufacturing capacity in the pharmaceutical sector in question, and then consider what political or economic repercussions may follow. More concrete alternatives for importation include parallel importation or the voluntary tiered-pricing arrangement proposed by the European Commission. Importantly, it is critical to monitor any public policy reform to assess whether or not they are achieving attendant outcomes and adjust accordingly. This will require baseline assessments and regular review at intervals.
The opportunities presented above can only be effective in addressing access to medicines in Ghana if other existing barriers are simultaneously addressed. First and foremost, the development and implementation of an effective exemption policy for the poor without co-payments is vital. Policies can vary such as implementing a national pricing policy that control prices on the supply side by regulating actual drug costs or the demand side, through reimbursement schemes such as reference-based pricing or generic substitution policies. Furthermore, reduction of mark-ups in the public sector may generate competition and drive private sector prices down. A hard but necessary policy reform is needed in the area of national tax, tariff and mark-ups to determine what changes could facilitate more affordable prices for the population.
Is the Ghana case generalisable for other African countries? We hope that as a minimum this case adds to the debate in other African countries about public policies they should pursue to improve access to medicines. Some policies may be more applicable than others depending on economic and political realities. There is not a "one-size-fits-all" policy menu that should be applied. Governments need to make informed policy choices when it comes to improving access to medicines and assess which measures are most needed and viable for their particular country.
Medecins Sans Frontieres (MSF): Fatal Imbalance: The Crisis in Research and Development for Drugs for Neglected Diseases. A report by the MSF Access to Essential Medicines Campaign and the Drugs for Neglected Diseases Working Group. Geneva. 2001, http://www.msf.org/source/access/2001/fatal/fatal.pdf
Google Scholar
Reich MR: The Global Drug Gap. Science. 2000, 287: 1979-81, 10.1126/science.287.5460.1979.
Article CAS PubMed Google Scholar
Cohen JC, Gyansa-Lutterodt M, Torpey K: Improving access to medicines: policy options for Ghana. Report prepared for the UK Department of International Development and the Government of Ghana. 2004
Ostrom BA: Increasing Access to Medicines: Health Systems Issues. Ghana Desk Based Consultancy, DFID Health Resource Centre. 2003
Management Sciences for Health (MSH): Strategies for Enhancing Access to Medicines (SEAM), Ghana: Key Findings. 2003, http://www.msh.org/seam/country-programs/3.1.3.htm
Cohen JC: Government and Market Failures in the Pharmaceutical System: Partial Explanations towards Understanding the Troubling Drug Gap. Proceedings of the Intellectual Property and International Public Health Conference, Washington, D.C. October 8 2003, http://www.iipi.org/conferences/IP&Health/cohen-paper.pdf
Cohen JC, Illingworth P: The Dilemma of Intellectual Property Rights for Pharmaceuticals: The Tension between Ensuring Access of the Poor to Medicines and Committing to International Agreements. Developing World Bioeth. 2003, 3: 27-48.
Article Google Scholar
Berger JM: Advancing Public Health by Other Means: Using Competition Policy to Mitigate the Impact of Patent Protection. Paper presented at the ICTS/UNCTAD/TIPS Regional Dialogue, Intellectual Property Rights, Innovation and Sustainable Development in Eastern and Southern Africa, Cape Town, South Africa. 29 June – 1 July 2004, http://www.iprsonline.org/unctadictsd/bellagio/docs/Berger_Bellagio3.pdf
Correa CM: Integrating Public Health Concerns into Patent Legislation in Developing Countries. 2000, Geneva, Switzerland: South Centre
World Trade Organization (WTO): Ministerial Declaration, 4th Session, Doha Ministerial Conference. 2001, WT/MIN(01)/DEC/2, http://www.wto.org/english/thewto_e/minist_e/min01_e/mindecl_e.pdf
The Separate Doha Declaration Explained. Undated. http://www.wto.org/english/tratop_e/trips_e/healthdeclexpln_e.htm
Correa CM: Implications of the Doha Declaration on the TRIPS Agreement and Public Health. Health Economics and Drugs EDM Series. 2002, 12: WHO/EDM/PAR/2002.3
Abbott FM: The Doha Declaration on the TRIPS Agreement and Public Health: Lighting a Dark Corner at the WTO. J Int Economic Law. 2002, 5: 469-505. 10.1093/jiel/5.2.469.
Reichman H, Hasenzahl C: Non-voluntary licensing of patented inventions: historical perspective, legal framework under TRIPS, and an overview of the practice in Canada and the USA. UNCTAD/ICTSD Project on IPRs and Sustainable Development. 2003, http://www.ictsd.org/pubs/ictsd_series/iprs/CS_reichman_hasenzahl.pdf
World Trade Organization (WTO): Implementation of paragraph 6 of the Doha Declaration on the TRIPS Agreement and public health. Decision of the General Council of 30 August 2003. General Council. 2003, WT/L/540, http://www.wto.org/english/tratop_e/trips_e/implem_para6_e.htm
World Trade Organization (WTO): The General Council Chairperson's statement. 2003, http://www.wto.org/english/news_e/news03_e/trips_stat_28aug03_e.htm
Republic of Ghana: Patent Law. 1992, P.N.D.C.L.305A
Republic of Ghana: Act 657, entitled the Patents Act, 2003, An Act to Provide for the Protection of Inventions and Other Related Matters. 2003
Cohen JC: Canada and Brazil Dealing with Tension between Ensuring Access to Medicines and Complying with Pharmaceutical Patent Standards: Is the Story the Same?. Working Paper for the Comparative Programme on Health and Society. 2003, http://www.utoronto.ca/cphs/WorkingPapers_2003-4.shtml
Outterson K: Pharmaceutical Arbitrage: Balancing Access and Innovation in International Prescription Drug Markets. Yale Journal of Health Policy, Law and Ethics. 2005, 5 (1):
Oxfam: Generic Competition, Price, and Access to Medicines: the Case of Antiretrovirals in Uganda. Oxfam Briefing Paper. 2002, http://www.oxfam.org.uk/what_we_do/issues/health/bp26_generic.htm
Velasquez G, Boulet P: Globalization and Access to Drugs: Perspectives on the TRIPS Agreement. World Health Organization Ref. No. WHO/DAP/98.9 Revised. 1999, http://www.who.int/medicines/library/dap/who-dap-98-9-rev/who-dap-98-9.shtml
Matthews D: WTO Decision on Implementation of Paragraph 6 of the Doha Declaration on the TRIPS Agreement and Public Health: a Solution to the Access to Essential Medicines Problem?. J Int Economic Law. 2004, 7: 73-107. 10.1093/jiel/7.1.73.
Cohen JC, Lybecker KM: AIDS Policy and Pharmaceutical Patents: Brazil's Strategy to Safeguard Public Health. The World Economy. 2005, 28 (2): 211-230. 10.1111/j.1467-9701.2005.00668.x.
World Health Organization (WHO): Globalization, TRIPS and access to pharmaceuticals. WHO Policy Perspectives on Medicines, No. 3. 2001, http://www.who.int/medicines/organization/ood/ood6pagers.shtml
Cohen JC: Canada's initiative to reform patent law for pharmaceuticals to help the poor. Can Pharm J. 2004, 137: 21-22.
European Commission: Council Regulation (EC) No 953/2003 of 26 May 2003 to avoid trade diversion into the European Union of certain key medicines. 2003, http://trade-info.cec.eu.int/cgi-bin/antitradediversion/index.pl
Correa CM: Recent International Developments in the Area of Intellectual Property Rights. ICTSD-UNCTAD Dialogue, 2nd Bellagio Series on Development and Intellectual Property. 2003, http://www.iprsonline.org/unctadictsd/bellagio/docs/Correa_Bellagio2.pdf
Government of Canada: Bill C-9, An Act to amend the Patent Act and the Food and Drugs Act (the Jean Chretien Pledge to Africa), 3rd Sess., 37th Parl, 52–53 Elizabeth II, 2004. 2004, http://www.parl.gc.ca/common/Bills_ls.asp?Parl=37&Ses=3&ls=C9
Ministry of Foreign Affairs, Norway: Regulations amending the Patent Regulations (in accordance with the decision of the WTO General Council of 30 August 2003, Paragraphs 1(b) and 2(a)). 2004, http://www.dep.no/ud/engelsk/p2500832/p30003923/032121-990069/dok-bn.html
Joint NGO Statement on TRIPS and Public Health WTO Deal on Medicines: A "Gift" Bound in Red Tape. 2003, http://www.cptech.org/ip/wto/p6/ngos09102003.html
United States Communication on Paragraph 6 of the Doha Declaration on TRIPS and Public Health. Undated. http://www.ustr.gov/Trade_Sectors/Intellectual_Property/Public_Health/Section_Index.html
Khor M: Intellectual Property, Biodiversity and Sustainable Development: Resolving the Difficult Issues. Zed Books, Third World Network. 2002
Management Sciences for Health (MSH): Managing Drug Supply: The Selection, Procurement, Distribution, and Use of Pharmaceuticals. 1997, Bloomfield, CT: Kumarian Press Inc
Download references
Acknowledgements
The Department for International Development (DfID), UK provided funds for the initial policy option analysis for increasing access to medicines in Ghana.
The authors declare that they have no competing interests. However, for the record, please note that Jillian Clare Cohen, Martha Gyansa-Lutterodt and Kwasi Torpey were DfID consultants in Ghana in 2003.
Author information
Authors and affiliations.
Leslie Dan Faculty of Pharmacy, University of Toronto, Munk Centre for International Studies, University of Toronto, Canada
Ghana National Drug Programme, Ministry of Health, Republic of Ghana
M Gyansa-Lutterodt
Family Health International, Canada
Faculty of Pharmaceutical Sciences, University of Toronto, Canada
Faculty of Law, University of Toronto, Canada
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to JC Cohen .
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Cohen, J., Gyansa-Lutterodt, M., Torpey, K. et al. TRIPS, the Doha Declaration and increasing access to medicines: policy options for Ghana. Global Health 1 , 17 (2005). https://doi.org/10.1186/1744-8603-1-17
Download citation
Received : 05 July 2005
Accepted : 09 December 2005
Published : 09 December 2005
DOI : https://doi.org/10.1186/1744-8603-1-17
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Essential Medicine
- Compulsory License
- Trips Agreement
- Patent Holder
Globalization and Health
ISSN: 1744-8603
- General enquiries: [email protected]
The Arguments For and Against the TRIPS Agreement
Following the culmination of the Uruguay Round of trade talks, the World Trade Organisation entered into existence on 1 January 1995. Alongside agreements on goods (GATT) and services (GATS), the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) forms one of the three pillars of the new multilateral trading system (WTO, 2008: 24). While it represents the first comprehensive and enforceable global agreement on Intellectual Property Rights (IPRs), it has since its inception been the subject of much criticism (Sell & Prakash, 2004). This paper outlines the main arguments both for and against TRIPS, and in so doing provides a sceptical assessment of its legitimacy and effectiveness. It begins, firstly, with the principal arguments in favour of TRIPS, before critically examining the recent history of the agreement and IPRs more generally. The paper then moves on to discuss the impact of TRIPS on economic development, and concludes that criticism of the agreement is broadly persuasive.
The WTO position
The standard line in support of TRIPS stems from recognition of the contemporary significance of the knowledge economy, and private intellectual property (IP) as a major component of international trade (WTO, 2008: 39). Disagreements over, and absence of, IPR protection constitute significant non-tariff barriers to trade, and TRIPS is the result of the need for a robust multilateral framework to replace what was an ineffective patchwork of pre-existing IPR agreements[i] (Matthews, 2002: 10-12). For the first time, therefore, TRIPS has put in place a global minimum standard of IP protection that all WTO members must adhere to. This covers copyrights, trademarks, industrial designs, geographical indications, patents, integrated circuit designs, trade secrets, and anti-competitive contract restrictions. Like other WTO agreements, it applies the fundamental principles of non-discrimination – most-favoured-nation treatment (no discrimination between trading partners) and national treatment (giving foreigners the same treatment domestically as one’s own nationals).
Various wider benefits to society are said to accrue from the imposition of temporary monopolies and other limitations that result from private IPRs (WTO, 2008: 39; CIPR, 2002: 14-18). By instituting legal protection – tackling piracy and counterfeiting – the disclosure of new knowledge and creativity is encouraged, and the significant costs associated with the creative process (such as with research and development) can therefore be recouped and remuneration earned. Innovation is thus both rewarded and further promoted. The scope and reliability offered by a global IPR regime should not only stimulate domestic innovation, but the security offered to developed world patent holders and others can also encourage foreign direct investment, technology transfer and licensing, and the diffusion of knowledge to the developing world (Matthews, 2002: 108-111). TRIPS is therefore able to play a significant role in the overall promotion of trade and economic development.
The agreement also takes care to recognise the differing position of member states vis-à-vis their relative economic status, administrative capabilities, and technological base. As per other WTO agreements, developing countries were afforded special and differential treatment as detailed in Part VI of the agreement under ‘transitional arrangements’. While developed countries had to ensure compliance by 1 January 1996, developing and post-communist countries were instead allocated a further four years to achieve this (with another five years granted for new patents products). Under Article 66.1, least-developed countries (LDCs) were given until 2006 to enact TRIPS, with the possibility of further extensions; the 2001 Doha Declaration on TRIPS and Public Health has also subsequently allowed a further ten years for pharmaceutical products for LDCs (WTO, 2001). Article 66.2 meanwhile explicitly encourages technology transfer from developed states to the LDCs so as to assist in the establishment of a viable technological base, and Article 67 obliges developed countries to provide technical and financial assistance to facilitate implementation of the agreement.
A further advantage inherent within TRIPS is the ‘flexibility’ offered to all members in interpreting various articles of the agreement (Vandoren, 2001). Article 27.3, for example, allows members to exclude certain inventions and subject matter from patentability, and permits the protection of others – such as plant varieties – through compatible sui generis systems. The Doha Declaration reiterated that developing countries have the right to grant compulsory licences or allow parallel importing for pharmaceutical products under Article 31 to tackle ‘national emergencies or other circumstances of extreme urgency’ – and that public health crises such as HIV/AIDS , malaria, and other epidemics can be declared as such (WTO, 2001).
Crucially, TRIPS also represents a significant improvement on previous IPR agreements in having considerable monitoring, enforcement, and dispute settlement capabilities (Matthews, 2002: 79-95). A TRIPS Council – comprising all WTO members – reviews national legislation and implementation of the agreement. Should serious disputes occur, any member may ultimately bring a case to the WTO’s Dispute Settlement Body, which has the power to issue punitive trade sanctions to ensure compliance. Successful cases launched by Ecuador and Brazil show that the dispute resolution mechanism works for both developed and developing countries alike (MIP, 2010). TRIPS is therefore seen by its supporters as representing an enforceable global system of IPR protection that plays an essential role in the modern global information society. By rewarding and encouraging innovation, it facilitates international trade, spurs economic growth, and enables technological progress and the dissemination of knowledge, ultimately benefiting both producers and users throughout the developed and developing world.
A critical perspective
Carla Hesse (2002: 26) usefully reminds us, however, that “the concept of intellectual property – the idea that an idea can be owned – is a child of the European Enlightenment”. Unlike physical property, knowledge is generally not rivalrous and can be shared without loss of utility: one person’s contemporaneous use of it does not detract from another’s ability to do so. The institution of intellectual property therefore involves the ‘construction of scarcity’ where none necessarily exists (May & Sell, 2006: 17-20). As noted above, granting of private IP rights is intended to protect and encourage innovation; a balance is consequently to be struck between the private rights of ownership and the public good of shared knowledge, for the broader welfare of society.
The TRIPS agreement is however predicated on a particular conception of intellectual property as an idea, and internationalising this can be problematic. This may be in the narrow sense that different societies afford greater priority to the public good on a variety of issues, and in the broader sense that some forms of ‘traditional knowledge’ (TK) as shared amongst indigenous communities do not conform to the codified Western model of individual and exclusive ownership (Michalopoulos, 2003: 17-18). The recent progress made in biotechnology-based products has notably highlighted this contrast: for Western advocates, modern genetic research aimed at increasing human welfare is entirely respectable ‘bioprospecting’, a form of IP that fits within the TRIPS framework. For indigenous peoples, by way of contrast, the patenting of TK resources such as neem extract[ii] can be seen as a form of ‘biopiracy’, and represents the “disingenuous repackaging of traditional knowledge in order to secure monopoly rents for the biopirate while excluding the original innovator from a claim of these rents” (Isaac & Kerr, 2004). TRIPS, crucially, does not currently provide an agreed interpretation of either what constitutes traditional knowledge, or how it should be protected (CIPR, 2002b: 73-87).
It is also vital to note that the concept of intellectual property as contained within the TRIPS agreement stems from a particular interpretation of IPRs that has developed within the Western tradition over the last few decades alone. Intellectual property was initially highlighted an international issue in the 1960s and 1970s by the G77 group of developing countries, when as part of the push for a New International Economic Order they unsuccessfully sought the dilution of existing IPR protection in order to narrow the technology gap with the developed world (May & Sell, 2006: 155-156). This debate, however, also galvanised various corporate actors in the USA – and to a lesser extent in Europe and Japan – who were becoming increasingly concerned about losses stemming from trade in counterfeit goods.
While pressure from the likes of the chemical, pharmaceutical, and entertainment industries led to a ‘silent revolution’ in stricter IPR protection within the USA from the 1970s onward, corporate lobbyists also sought to move the issue to the global level (Archibugi & Filippetti, 2010). American government policy-makers, concerned about the country’s trade deficit and loss of competitiveness, became increasingly receptive to their lines of argument. International IPRs as based on protection and exclusion, rather than competition and diffusion, were thus promoted as a means of maintaining a comparative advantage in emerging knowledge-based, high-technology sectors of the global economy. Business groups succeeded in placing IP protection on the Uruguay Round agenda, and – through claiming unrivalled expertise in arcane ‘technical’ matters of IP legislation – they managed to play a key role in shaping the terms of the agreement (Matthews, 2002: 7-28).
Developing countries, on the other hand, had little input, although while many were initially sceptical of the proposals, resistance to a deal on TRIPS was gradually overcome (Drahos, 2002). The promise of greater access to agricultural and textile markets, economic coercion via threat of American sanctions, the potential development of restrictive bilateral IPR agreements, and an overall lack of awareness of the content of proposals, all played their part (May & Sell, 2006: 157-158). TRIPS therefore reflected the interests of particular global corporate actors, and it is only through acknowledgement of this that it can be properly understood (Matthews, 2002: 4-5). It is the specific view of intellectual property as promoted by Western transnational corporations, and supported by governments, that became embedded within the agreement. The fine balance between private gain and the public good – the purpose and scope of IPRs – may therefore have been tilted too far toward the former at the expense of the latter, and the consequences of this for how TRIPs functions in spite of its stated objectives are what we now turn to.
TRIPS and development
One of the principal criticisms made of the TRIPS agreement is that it offers an inappropriate uniform standard across a diverse range of states. Developed countries generally already possess suitable levels of IPR protection, and are home to the overwhelming majority of IP rights-holders that stand to benefit from increased protection (Chang, 2001: 23). Most developing countries, on the other hand, may incur significant costs from raising domestic standards to the required level – taking scarce resources away from other crucial sectors – and from the increased payments to be made to developed world rights-holders. A 2001 World Bank report suggested that in the short term TRIPS effectively constitutes an annual $20 billion transfer of wealth from technology-importing developing countries to technology-exporting developed countries (cited in Dutfield & Suthersanen, 2004). Similarly, Philip McCalman (2005) estimates that the beneficiaries of TRIPS are just a handful of developed countries: primarily the USA and various Western European states. Countries from India to Brazil – and even South Korea – meanwhile suffer through their reliance on technology imports. While most countries could still benefit in the long-term, McCalman argues that the advantages will nonetheless be distributed overwhelmingly amongst the leading developed countries. The Commission on Intellectual Property Rights also arrived at similar conclusions in its 2002 report on IPRs and development – assessing the argument that a stronger IPR regime would offset short-term implementation costs over the long-term, they concluded that:
“…for most developing countries with weak technological capacity, the evidence on trade, foreign investment, and growth suggests IP protection will have little impact. Nor is it likely that the benefits of IP protection will outweigh the costs in the foreseeable future. For more technologically advanced developing countries, the balance is finer. Dynamic gains may be achieved through IP protection, but at cost to other industries and consumers.”
(CIPR, 2002a: 12)
The Commission emphasised that developing countries do require different IP strategies as depending on their level of development. Contrary to the claims of TRIPS advocates, “rapid growth is more often associated with weaker IP protection” and does not start to become important until a country is well into upper-middle income category (CIPR, 2002b:22). So, while the WTO may claim to take developing countries needs into account, its emphasis on the need for a single high standard of IPRs appears to run contrary to the historical evidence. Indeed, Ha-Joon Chang observes of Europe and the USA that they themselves were “still routinely violating the IPRs of other countries’ citizens well into the twentieth century” (2001: 10). The flexible use of IP regimes to further economic interests was also successfully adopted by East Asian states such as Korea and Taiwan until very recently, where imitation and reverse-engineering were all considered important methods for developing technological and innovative capacity (CIPR, 2002b: 19-20). Strong IPR protection therefore appears to be a consequence, rather than a cause, of economic development.
The potential social cost of TRIPS for poorer countries has meanwhile been particularly evident over the issue of access to medicines, most notably with regard to antiretroviral drugs (Lanoszka, 2003). Before TRIPS, many countries either did not patent medicines or provided less than the robust 20-year protection subsequently introduced. Contemporary TRIPS rules, however, drive up costs to unaffordable levels by enabling monopoly pricing and excluding cheaper ‘generic’ alternatives. In 2001, by way of example, a group of 39 pharmaceutical companies took the South African government to court to prevent their use of compulsory licensing of generics, although intense public pressure did eventually force them to abandon the case (Sell & Prakash, 2004). However, the incident showed how multinational corporations attempt to use TRIPS to pursue private gain at clear cost to the public good[iii]. While some flexibilities in interpreting the agreement in light of public health concerns have since been established, as mentioned above, they often remain unused owing to cost, complexity, and the threat of trade ‘retaliation’ (O’Farrell, 2008).
The few allowances that are granted to developing countries in the TRIPS agreement can therefore be seen as insufficient, and the numerous limitations far too restrictive. As Constantine Michalopoulos (2003) notes, TRIPS does not actually offer the same range of ‘special and differential treatment’ (SDT) as other WTO agreements. Once the transition periods have expired, developing countries must implement the same rules on scope and duration of protection – regardless of circumstances – as the most advanced developed countries[iv]. Any form of permanent SDT is not an option: LDCs can no longer exempt sectors from protection, or reduce patent duration, as a means of addressing social or economic concerns. The flexibilities within the agreement, as seen above, therefore offer only limited room for manoeuvre.
Robert Wade (2003) suggests that as a result this constitutes a distinct ‘shrinking of the development space’: a reduction in states’ policy-making autonomy that denies them the paths to development that were taken by others before them. Furthermore, the agreement is “vague at points where vagueness benefits the developed countries, and precise at points where precision works against developing countries” (Wade, 2003: 630). The obligations of developing countries and the rights of developed are both enforceable to a far greater extent than the rights of the developing and the obligations of the developed. There is, for example, despite the clearly stated objective of Article 66.2 regarding technology transfer, little evidence of a sustained effort by developed states to honour such commitments (Moon, 2008).
The legitimacy and effectiveness of the TRIPS agreement is clearly vulnerable to numerous criticisms, particularly so with regard to developing countries. It is noteworthy that even prominent free trade advocates such as Martin Wolf (2005: 217) criticise the ‘hypocrisy’ of TRIPS, seeing it as a rent extraction device for many developing countries, with potentially devastating effects on education, public health, and economic development. Even within those countries who appear to gain most from the agreement, the benefits may only accrue to particular sections of society, so that “the real winners from TRIPS are not advanced countries, but rather the large corporations that pressed for its adoption” (Archibugi & Filippetti, 2010: 144). TRIPS has also not provided a solution to policy-makers’ concerns, as trade balances have continued to erode, while the recent emphasis on private rights may even serve to inhibit innovation and the spread of knowledge in developed countries in the long term (Hesse, 2002). While Archibugi & Filippetti (2010) caution against attributing too much importance to TRIPS, it is apparent that the agreement does not function as advertised. From a global perspective, it seems clear that adopting a ‘one-size-fits-all’ approach to IPRs is entirely inappropriate. A tiered system, offering more substantive special and differential treatment according to countries’ developmental needs, would have been more suitable. However, it remains to be seen whether major reform of the agreement is likely, given that TRIPS is now firmly established within the WTO system.
Bibliography
Archibugi, D. and Filippetti, A. (2010) ‘The Globalisation of Intellectual Property Rights: Four Learned Lessons and Four Theses’, Global Policy , 1, 2, 137-149.
Botov, I. E. (2004) ‘From the Paris Convention to the TRIPS Agreement: A One-Hundred-and-Twelve-Year Transitional Period for the Industrialized Countries’, Journal of World Intellectual Property , 7, 1, 115-130.
Chang, H-J. (2001) Intellectual Property Rights and Economic Development – Historical Lessons and Emerging Issues . Third World Network: Penang.
Commission on Intellectual Property Rights (CIPR) (2002a) Integrating Intellectual Property Rights and Development Policy: Executive Summary . CIPR: London.
Commission on Intellectual Property Rights (CIPR) (2002b) Integrating Intellectual Property Rights and Development Policy: Full Report . CIPR: London.
Drahos, P. (2002) ‘Developing Countries and International Intellectual Property Standard-Setting’, Journal of World Intellectual Property , 5, 5, 765-789.
Dutfield, G. and Suthersanen, U. (2004) ‘Harmonisation or Differentiation in Intellectual Property Protection? The Lessons of History’, Occasional Paper 15 , Quaker United Nations Office: Geneva. [Online] http://www.quno.org/geneva/pdf/economic/Occassional/Harmonisation-or-Differentiation.pdf, accessed 10 March 2011.
Hesse, C. (2002) ‘The rise of intellectual property, 700 B.C. – A.D. 2000: an idea in the balance’, Daedalus , Spring 2002, 26-45.
Isaac, G. E. and Kerr, W. A. (2004) ‘Bioprospecting or biopiracy? Intellectual Property and Traditional Knowledge in Biotechnology Innovation’, Journal of World Intellectual Property , 7, 1, 35-52.
Lanoszka, O. (2003) ‘The Global Politics of Intellectual Property Rights and Pharmaceutical Drug Policies in Developing Countries’, International Political Science Review , 24, 2, 181-197.
Managing Intellectual Property (MIP) (2010) Peter Drahos: How TRIPs reshaped IP norms. 1 st June 2010 p44 .
Matthews, D. (2002) Globalising Intellectual Property Rights: The TRIPs Agreement . Routledge: London.
May, C. and Sell, S. K. (2006) Intellectual Property Rights: A Critical History . Lynne Rienner Publishers: Boulder.
Michalopoulos, C. (2003) ‘Special and Differential Treatment of Developing Countries in TRIPS’, TRIPS Issues Paper 2 , Quaker United Nations Office: Geneva. [Online]
http://www.quno.org/geneva/pdf/economic/Issues/Special-Differential-Treatment-in-TRIPS-English.pdf, accessed 10 March 2011.
O’Farrell, G. (2008) ‘One Small Step or One Giant Leap Towards Access to Medicines for All?’, European Intellectual Property Review , 30, 6, 211-215.
Sell, S. K. and Prakash, A. (2004) ‘Using Ideas Strategically: The Contest Between Business and NGO networks in Intellectual Property Rights’, International Studies Quarterly , 48, 143-175.
Vandoren, P. (2001) ‘The TRIPS Agreement: A Rising Star’, Journal of World Intellectual Property , 4, 3, 307-322.
Wade, R. (2003) ‘What strategies are viable for developing countries today? The World Trade Organization and the shrinking of ‘development space’, Review of International Political Economy , 10, 4, 621-644.
Wolf, M. (2005) Why Globalization Works . Yale University Press: London.
World Trade Organisation (WTO) (2001) ‘Declaration on the TRIPS Agreement and Public Health’, World Trade Organisation. [Online] http://www.wto.org/english/thewto_e/minist_e/min01_e/mindecl_trips_e.htm, accessed 10 March 2011.
World Trade Organisation (WTO) (2008) Understanding the WTO . World Trade Organisation: Geneva.
World Trade Organisation (WTO) (2011) ‘Frequently asked questions about TRIPS in the WTO’, World Trade Organisation. [Online] http://www.wto.org/english/tratop_e/trips_e/tripfq_e.htm, accessed 10 March 2011.
[i] The main agreements – the Paris and Berne Conventions of 1883 and 1886 respectively – have been subsumed within TRIPS, as have parts of the 1961 Rome Convention and the 1989 Washington Treaty (WTO, 2011).
[ii] Neem is a tree from South Asia whose extract has long been used as a form of natural medicine, pesticide, and fertilizer (CIPR, 2002b: 76)
[iii] As Chang (2001: 15-16) notes, the industry argument that profit through such strict patent protection is critical for innovation remains unconvincing. Scientific curiosity and common humanity are often sufficient motivation, while public funds are often provided to assist private sector research, and profits can also still accrue via ‘natural protective mechanisms’ (e.g. imitation lag and reputational advantage).
[iv] Botov (2004) describes how developed countries had over a century – from the Paris Convention in 1883 to the WTO agreement in 1995 – to fully develop their IPR regimes, and suggests the same latitude should be extended to today’s developing countries.
Written by: Ben Willis Written at: University of Plymouth Written for: Piers Revell Date written: March 2011
Further Reading on E-International Relations
- TRIPS-Plus Provisions and the Access to HIV Treatments in Developing Countries
- Poliheuristic Analysis: 2008 Indo-US Civil Nuclear Agreement
- Indian Perspective on Iran-China 25-year Agreement
- A Pareto Optimal Peace: How the Dayton Peace Agreement Struck a Unique Balance
- Intermestic Realism: Domestic Considerations in International Relations
- Is Universal Health Coverage Always the Best Solution to Health Challenges?
Please Consider Donating
Before you download your free e-book, please consider donating to support open access publishing.
E-IR is an independent non-profit publisher run by an all volunteer team. Your donations allow us to invest in new open access titles and pay our bandwidth bills to ensure we keep our existing titles free to view. Any amount, in any currency, is appreciated. Many thanks!
Donations are voluntary and not required to download the e-book - your link to download is below.
Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS Agreement)
TextileFuture
By virginia, applications invited for wto workshop on article 67 of trips agreement.
The WTO Intellectual Property (IP) Division has opened the application process for a workshop on Article 67 of the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) to be held at the WTO on 12-13 June 2023. To help facilitate implementation of the TRIPS Agreement, Article 67 requires developed country members to provide, on request and on mutually agreed terms and conditions, technical and financial cooperation in favour of developing and least developed country (LDC) members. The deadline to submit applications is April 21, 2023.

What is the purpose of the workshop?
The workshop will facilitate exchange on prevailing trends in the sphere of IP-related assistance. Drawing upon Article 67 of the TRIPS Agreement , the event will aim to enhance dialogue between beneficiary and assistance-providing members and intergovernmental organizations (IGOs), with a view to improving overall coordination and the matching of programmes with priority needs.
The workshop will also provide an opportunity for participants to assist in the design and delivery of tailored and coordinated capacity building activities that respond to needs identified by beneficiary members and observers.
Who can apply?
Government officials from members and observers eligible to benefit from WTO training activities, with responsibility for, or direct experience in managing, intellectual property and the reception of IP-related technical assistance programmes are invited to apply.
Applicants should have advanced knowledge of one of the three official languages of the WTO, including working proficiency in English sufficient to permit active participation in small group discussions.
How to apply?
Government officials from developing and LDC members and observers eligible to benefit from WTO training activities are invited to apply by April 21, 2023. A total of 30 participants will be accepted for the workshop.
Detailed information regarding the workshop and application process is available here .
www.wto.org
Share this TextileFuture content with:
- Click to email a link to a friend (Opens in new window)
- Click to print (Opens in new window)
- Click to share on Facebook (Opens in new window)
- Click to share on Twitter (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
WTO/GATT Research: Citing the WTO agreements
- Selected books, databases & websites
- Books, Ebooks, Working papers, etc.
- Law reviews, journals, articles
- News, blogs & paper/note topics
- Status of the WTO Agreements
- Citing the WTO agreements
- Additional sources of the WTO agreements & other trade agreements
- Drafting & negotiating history
- WTO structure, members, meetings
- Schedules, tariffs & non-tariff measures
- GATT and early WTO documents, 1940-2000
- WTO documents, 1995-present
- Citing official U.S. sources
- U.S. regulations & tariff schedules
- U.S. government websites, etc.
- U.S. legislative history
- Rules, procedure, process, etc.
- Find disputes by topic, article number, etc.
- Dispute settlement reporters & citation
- Documents & submissions in dispute settlements
- Selected books about dispute settlement
- Trade Policy Reviews
- Statistics & terminology
- Other research guides
Bluebook citation of the WTO Agreements
To cite a multilateral convention, Rule 21.4.5(a)(ii) of The Bluebook: A Uniform System of Citation , 20th ed., requires a U.S. source, if therein, with an optional parallel citation to a multilateral source. If neither of those can be obtained, Rule 21.4.5(c) requires a cite to I.L.M. or another unofficial source. Rule 21.11(d) has examples for some WTO and former GATT-era agreements. NOTE: Bluebook rules can change, so please check the latest edition at the time of your writing.
The suggested citations below do not contain any U.S. treaty sources because the U.S. did not ratify the Uruguay Round agreements as treaties, but instead implemented them through legislation and administrative action. See Citing official U.S. sources .
Instead, the citations below use U.N.T.S. and I.L.M. To locate U.N.T.S. see here and I.L.M. here . Unlike the United Nations, there is no separate GATT or WTO treaty series. In less recent publications, you may also see a citation to a WTO publication called The Legal Texts: The Results of the Uruguay Round of Multilateral Trade Negotiations (Cambridge, U.K.; New York, N.Y.: Cambridge University Press, [2010] 1999) Reserve K4600.A35 R473 2010 .
SUGGESTED CITATIONS:
Final Act Embodying the Results of the Uruguay Round of Multilateral Trade Negotiations, Apr. 15, 1994, 1867 U.N.T.S. 14, 33 I.L.M. 1143 (1994) [hereinafter Final Act].
WTO Agreement: Marrakesh Agreement Establishing the World Trade Organization, Apr. 15, 1994, 1867 U.N.T.S. 154, 33 I.L.M. 1144 (1994) [hereinafter Marrakesh Agreement or WTO Agreement].
GATT 1994:General Agreement on Tariffs and Trade 1994, Apr. 15, 1994, Marrakesh Agreement Establishing the World Trade Organization, Annex 1A, 1867 U.N.T.S. 187, 33 I.L.M. 1153 (1994) [hereinafter GATT 1994].
Agreement on Agriculture, Apr. 15, 1994, Marrakesh Agreement Establishing the World Trade Organization, Annex 1A, 1867 U.N.T.S. 410. [Not reproduced in I.L.M.]
TRIMS Agreement: Agreement on Trade-Related Investment Measures, Apr. 15, 1994, Marrakesh Agreement Establishing the World Trade Organization, Annex 1A, 1868 U.N.T.S. 186 [hereinafter TRIMS Agreement]. [Not reproduced in I.L.M.]
Agreement on Subsidies and Countervailing Measures, Apr. 15, 1994, Marrakesh Agreement Establishing the World Trade Organization, Annex 1A, 1869 U.N.T.S. 14. [Not reproduced in I.L.M.]
GATS: General Agreement on Trade in Services, Apr. 15, 1994, Marrakesh Agreement Establishing the World Trade Organization, Annex 1B, 1869 U.N.T.S. 183, 33 I.L.M. 1167 (1994) [hereinafter GATS].
TRIPS: Agreement on Trade-Related Aspects of Intellectual Property Rights, Apr. 15, 1994, Marrakesh Agreement Establishing the World Trade Organization, Annex 1C, 1869 U.N.T.S. 299, 33 I.L.M. 1197 (1994) [hereinafter TRIPS Agreement].
DSU, Dispute Settlement Rules: Understanding on Rules and Procedures Governing the Settlement of Disputes, Marrakesh Agreement Establishing the World Trade Organization, Annex 2, 1869 U.N.T.S. 401, 33 I.L.M. 1226 (1994) [hereinafter DSU]
U.S. sources for the WTO Agreements & the GATT 1947
See Citing official U.S. sources in this guide.
- << Previous: Status of the WTO Agreements
- Next: Additional sources of the WTO agreements & other trade agreements >>
- Last Updated: Aug 9, 2024 11:35 AM
- URL: https://nyulaw.libguides.com/wto_gatt
ARTICLE 67 TRIP REDUCTION
25-67-1: intent and purpose:.
Use of the private automobile in southern California has created serious traffic congestion and air quality problems. At the regional and local levels, government agencies have adopted policies and programs aimed at reducing the number of single passenger vehicles traveling along the region’s highways and thereby alleviating congestion and improving air quality. The trip reduction measures contained in this article are intended to help meet regional congestion management and air quality goals. (Ord. #1563, §3)
25-67-2: PARKING AND LOADING REQUIREMENTS:
In addition to the parking requirements contained in article 65 of this chapter, the following parking standards shall apply:
A. Carpool/Vanpool Parking; Office Development And Business Parks: For all new developments in the M zoning district, a minimum of three (3) parking spaces shall be reserved for use by carpool/vanpool users for each fifty (50) total spaces. The carpool/vanpool spaces shall be located in close proximity to each building in an office or business park facility. Carpool/vanpool spaces shall be included in the total parking count as shown in the example below:
All carpool/vanpool spaces shall be signed to identify them as reserved for these users, and shall be designed to accommodate full sized passenger vans.
B. Residential Project Requirements: For projects consisting of ten (10) or more attached apartment or condominium units, the following requirements shall apply:
1. Each building shall be directly accessible to a public roadway via sidewalks or paved walkways.
2. Each project providing one hundred (100) or more parking spaces shall provide a passenger loading area in close proximity to the main entrance to each building. This area shall be equal in size to five (5) standard parking spaces. (Ord. #1563, §3)
25-67-3: TRANSIT FACILITIES:
Transit facilities such as bus turnouts, bus stops and shelters, and bus pads shall be required if determined to be necessary by the planning director. (Ord. #1563, §3)
25-67-4: VIDEO CONFERENCING:
One on site video conferencing facility shall be provided in each office building of two hundred thousand (200,000) square feet or larger in size. In an office development where no single building exceeds two hundred thousand (200,000) square feet, but the aggregate of all buildings exceeds three hundred thousand (300,000) square feet, one on site video conferencing facility accessible to tenants from each building shall be provided. Video conferencing facilities shall provide the capability to provide voice and data connections with other video conferencing facilities. (Ord. #1563, §3)
25-67-5: TELECOMMUTING CENTERS:
Each single-family subdivision consisting of five hundred (500) or more homes or each mixed use project containing five hundred (500) or more homes shall either provide one telecommuting center or an in lieu payment to the city sufficient to construct and staff such a center for a period of at least five (5) years. (Ord. #1563, §3)
25-67-6: CERTIFICATE OF OCCUPANCY:
A. For nonresidential uses, all facilities and improvements required by this article shall be constructed or otherwise provided prior to the issuance of a certificate of occupancy for any new building or addition to an existing building subject to the requirements of this article.
B. For residential uses, all facilities and improvements required by this article shall be provided prior to the issuance of any certificates of occupancy for more than the first half of the total number of dwelling units. For example, in a project which includes six hundred (600) single-family homes, required facilities and improvements would need to be provided prior to the issuance of certificates of occupancy for units three hundred one (301) and above. (Ord. #1563, §3)
Search form
- Publications
- Get Involved
- Planned Giving
Russian Offensive Campaign Assessment, August 28, 2024

Christina Harward, Nicole Wolkov, Angelica Evans, Karolina Hird, and George Barros
August 28, 2024, 7pm ET
Click here to see ISW’s interactive map of the Russian invasion of Ukraine. This map is updated daily alongside the static maps present in this report.
Click here to see ISW's interactive map of Ukraine's offensive in Kursk Oblast.
Click here to see ISW’s 3D control of terrain topographic map of Ukraine. Use of a computer (not a mobile device) is strongly recommended for using this data-heavy tool.
Click here to access ISW’s archive of interactive time-lapse maps of the Russian invasion of Ukraine. These maps complement the static control-of-terrain map that ISW produces daily by showing a dynamic frontline. ISW will update this time-lapse map archive monthly.
Note: The data cut-off for this product was 12:30pm ET on August 28. ISW will cover subsequent reports in the August 29 Russian Offensive Campaign Assessment.
Multiple reports from Western media indicate that the US government is prohibiting the United Kingdom (UK) from allowing Ukraine to use Storm Shadow missiles to strike military targets in Russia. The Financial Times (FT) reported on August 27 that a source familiar with the matter stated that Ukraine's use of British and French Storm Shadows may require access to American intelligence, surveillance, and reconnaissance in areas where Russian forces are jamming the GPS signals that the missiles use for targeting.[1] FT reported that "well-placed" sources stated that the UK government sent a request to both the US and France earlier in summer 2024 to grant Ukraine permission to use Western-provided weapons to strike military targets in Russia, and French President Emmanuel Macron stated in May 2024 that Ukraine should be allowed to strike military sites in Russia from which Russian forces attack Ukraine. The Telegraph reported on August 27 that the UK government supports Ukraine's ability to strike military targets in Russia with Storm Shadow missiles but that the missiles also use unspecified, classified American systems, whose use requires US permission.[2] The Telegraph stated in a since-deleted section of its original web article that the UK has not formally asked the US to allow Ukraine to use Storm Shadows to strike military targets within Russia, and that a White House source stated that the US is concerned about how the use of the missiles — even without US approval — could escalate the situation and draw the US into the war in Ukraine. The Telegraph reported that British Prime Minister Keir Starmer is taking a "consultative approach" to negotiations with the US and does not want to spark a disagreement over the issue. A source in the UK government reportedly stated that Russia is aware that Ukraine is asking for permission to strike military targets in Russia, so Russia has moved its "most critical assets" out of range of long-range missile systems. ISW continues to assess that although Russian forces have moved aircraft out of range of Western-provided Storm Shadow and ATACMS missiles, a significant number of Russian military objects remain within striking distance of Western weapons, restrictions on which are allowing Russian forces to leverage sanctuary space in deep rear areas within Russia to support military operations against Ukraine.[3]
Several Russian milbloggers claimed on August 28 that the tempo of Ukrainian attacks in Kursk Oblast has slowed and that Ukrainian forces are now attempting to dig in and hold select areas they recently seized. These milbloggers claimed the intensity of Ukrainian attacks within Kursk Oblast has decreased and that Ukrainian forces are attempting to hold and fortify select areas, amid continued Ukrainian offensive operations within the salient in Kursk Oblast.[4] Other milbloggers claimed that Russian forces are gradually stabilizing the situation in Kursk Oblast.[5] Geolocated footage published on August 28 showing Russian forces operating in eastern Korenevo indicates that Ukrainian forces likely recently withdrew from the area and that Russian forces regained some lost positions.[6] A Russian milblogger claimed on August 28 that Ukrainian forces advanced past Vetreno, Kremyanskoye, and Sheptukhovka (all east and northeast of Korenevo); within western Nechayev and eastern Cherkasskoye Porechnoye (both northeast of Sudzha); and in the fields south of Spalnoye (southeast of Sudzha).[7] Russian milbloggers claimed that elements of the Russian 810th Naval Infantry Brigade (Pacific Fleet, Eastern Military District [EMD]) cleared Spalnoye and that Russian forces have regained control of the settlement.[8] The Russian Ministry of Defense (MoD) and other Russian sources claimed that Ukrainian forces also attacked east of Korenevo near Olgovka, north of Sudzha near Malaya Loknya, east of Sudzha near Russkaya Konopelka, and southeast of Sudzha near Borki.[9] A Russian milblogger claimed on August 28 that Russian forces repelled Ukrainian attempts to cross the international border near Zhuravlevka, Belgorod Oblast (southwest of Belgorod City and north of Kharkiv City).[10]
Kremlin Spokesperson Dmitry Peskov denied reports on August 28 that Russian conscripts are fighting in Kursk Oblast and called such reports a "distortion of reality," despite a plethora of evidence, including Russian evidence and admissions, to the contrary.[11] Chechen Republic Head Ramzan Kadyrov, Chechen Akhmat Spetsnaz Commander Apty Alaudinov, and other Russian sources have notably acknowledged that Russian conscripts are fighting in Kursk Oblast.[12] Russian opposition outlet Horizontal 7x7 reported on August 28 that Kremlin-controlled social media site VKontakte (VK) removed a local Ivanovo Oblast news outlet's post claiming that the Russian military is sending Airborne Forces (VDV) conscripts to Kursk oblast.[13] Horizontal 7x7 noted that the Ivanovo Oblast Human Rights Ombudsman previously stated that a conscript from Ivanovo Oblast returned to Russia during a prisoner-of-war exchange.[14] Russian opposition outlet Mobilization News reported that the Russian military plans to deploy Russian conscripts from the 290th Missile Regiment (7th Missile Corps, 27th Missile Army, Strategic Missile Forces) and 2187th VDV Regiment (98th VDV Brigade) to Kursk Oblast.[15]
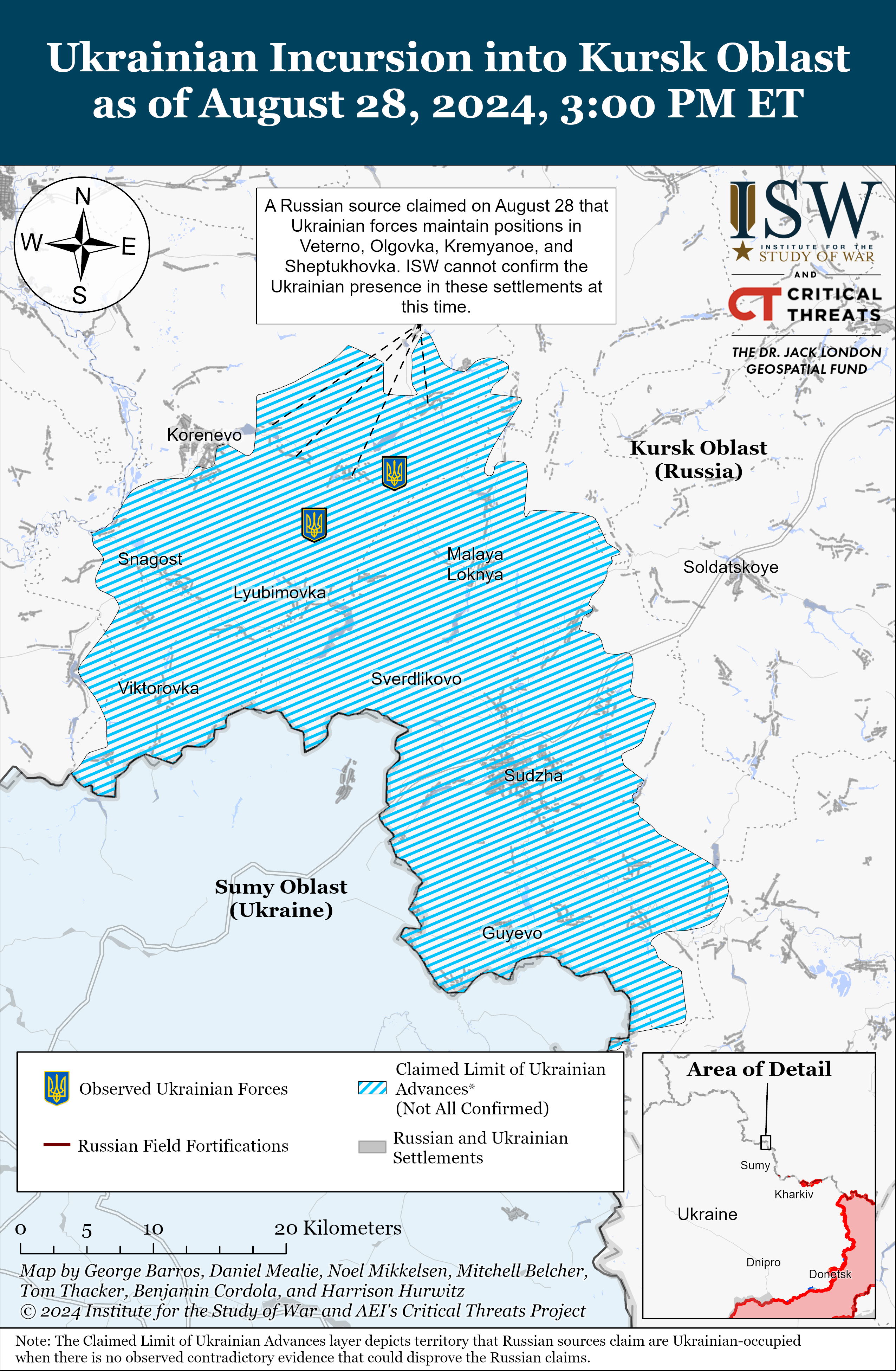
Ukrainian forces conducted drone strikes against oil infrastructure in Rostov and Kirov oblasts and reportedly conducted a drone strike against Voronezh Oblast overnight on August 27 to 28. Ukrainian outlet Suspilne reported that sources within the Ukrainian special services stated that Ukraine's Main Military Directorate (GUR) and Special Operations Forces conducted a strike with Ukrainian-made drones against the Atlas oil depot in Rostov Oblast.[16] Suspilne reported that the Atlas oil depot is part of the Russian defense industrial base (DIB) and supplies Russian forces. Rostov Oblast Governor Vasily Golubev claimed that a drone strike caused a fire at a fuel depot in Kamensky Raion.[17] A prominent Russian milblogger claimed that wreckage of one of the downed drones fell near the oil depot, starting a fire in one of the technical buildings that spread to tanks containing flammable materials.[18] Suspilne also reported that GUR conducted a strike with Ukrainian-made drones against the "Zenit" oil depot near Kotelnich, Kirov Oblast — the first time Ukrainian drones have struck Kirov Oblast, which is more than 1,200 kilometers from the Ukrainian border.[19] Suspilne stated that the "Zenit" oil depot provides fuel to the Russian military. Kirov Oblast Governor Alexander Sokolov claimed that Russian forces shot down two drones but that a third drone fell on a plant in Kotelnich and started a fire.[20] Russian state news outlet RIA Novosti reported that regional dispatch services stated that there was a small fire at the "Vyatka" Federal State Institution in Kotelnich that stores oil and refined products.[21] Voronezh Oblast Governor Alexander Gusev claimed that Russian forces destroyed a drone in Rossoshansky Raion and that falling drone debris caused a fire near explosive objects but there was no detonation.[22] A prominent milblogger claimed that Ukrainian forces were likely targeting the Minudobreniya agricultural chemical manufacturing plant south of Rossosh.[23] The Russian Ministry of Defense (MoD) claimed that Russian forces destroyed eight drones over Voronezh Oblast and four over Rostov Oblast overnight.[24] Russian sources denied claims on August 28 that there was also a fire at the Ryazan State District Power Plant in Pronsky Raion, Ryazan Oblast, alleging that dry grass caught fire on August 27.[25]
The prominent Kremlin-linked Rybar Telegram project's continued meetings with Iraqi officials appear to be supporting Iranian-backed Iraqi efforts to gain greater control of their information and media space. The Rybar team announced on August 28 that they met with the head of the media service of Iraq's Popular Mobilization Forces (PMF) Muhhanad al Aqaabi to discuss the role of foreign media organizations in Iraq's information environment.[26] Iran has notably co-opted the PMF and uses the organization to covertly enable its objectives in Iraq.[27] Rybar claimed that al Aqaabi spoke about Western media "propaganda" and information space manipulations during the war against the Islamic State of Iraq and al Sham (ISIS), particularly in 2015 to 2017, and praised Russian and Chinese media for reporting "impartially" on the war with ISIS. The Iraqi Communications and Media Commission (controlled by Iranian-aligned factions within Iraq), has taken efforts over the past year to institute media and information space controls to stifle opposition voices.[28] Russia has undertaken its own multifaceted and multidomain efforts to similarly restrict and control domestic media and information space, including by co-opting well-known media voices such as the Rybar project.[29] Iranian-backed Iraqi political actors may seek to learn similar tactics from Rybar and other Russian sources as they continue efforts to crack down on opposition in the information space.
Kremlin newswire TASS will soon open an office in Iran, supporting Moscow's efforts to deepen its partnership with Tehran. [30] TASS Director General Andrei Kondrashov announced on August 28 that TASS plans to open a correspondent office in an unspecified location in Iran, adding to the 62 international offices that TASS has in 57 countries.[31] Russia and Iran have deepened their military, economic, financial, technical, industrial, and political control over the backdrop of Russia's full-scale invasion of Ukraine, and TASS 's expansion into Iran is likely intended in part to extend that cooperation into the media sphere.[32] TASS 's expansion into Iran indicates growing media cooperation between Moscow and Tehran as well.
Russia's allies appear to be increasingly restricting their transactions with Russian companies and financial institutions amid ongoing concern about the impact of secondary US sanctions. Kremlin-affiliated business outlet Kommersant reported on August 28 that "market participants" stated that banks in the United Arab Emirates (UAE), specifically in Dubai, began blocking payments for electronic components and equipment from Russian companies in August 2024.[33] Kommersant noted that the UAE has previously served as a payment point between Russian importers and electronics companies in the People's Republic of China (PRC), but that UAE banks are growing increasingly concerned about the risk of secondary sanctions. General Director of Russian Beshtau Electronics company Oleg Osipov confirmed to Kommersant that the issues with the banks in Dubai began roughly at the end of July to the beginning of August 2024 and claimed that the "PRC side" is initiating the blocking, but did not provide additional details. Another source told Kommersant that Russian companies previously paid for PRC electronics via banks in the UAE, although the goods themselves were immediately sent to Russia, and that the UAE banks were accepting a commission of only 1–3 percent on the transactions. UAE electronic company Jacky's Electronics founder Guseyn Imanov told Kommersant that he is also aware of issues with paying for consumer electronics and components through banks in Dubai and claimed that Russian companies have found an unspecified alternative way to conduct financial transactions at an increased commission. Russian and Kyrgyz media recently reported that at least 12 Kyrgyz commercial banks have suspended personal monetary transfers through Russian banks, including Sberbank, T-Bank, and MTS Bank, for an indefinite period.[34] Kyrgyzstan's National Bank told Kyrgyz outlet 24.kg that commercial banks in Kyrgyzstan are taking measures to prevent negative consequences of international sanctions.[35] The US Department of Treasury's Office of Foreign Assets Control (OFAC) issued a series of additional sanctions on August 23 against Russian and Chinese companies and actors accused of being involved in Russia's war effort and supporting Russia's defense industry.[36] Russia's allies appear to be responding more strongly to these more recent sanctions, and additional foreign banks may take steps to avoid the wider risk of secondary US sanctions in the coming weeks and months.
The Russian Federal Security Service (FSB) reportedly prevented a terrorist attack in the Republic of Ingushetia on August 28. The FSB detained six residents of the Republic of Ingushetia on August 28 for preparing sabotage and terrorist attacks on law enforcement officers and a Russian Orthodox church in Sunzha.[37] The FSB claimed that "supporters of international terrorist organizations" contacted one of the suspects, who received orders from a militant in Syria to attack the church.[38] The Investigative Committee for the Republic of Ingushetia opened a criminal case against three residents for participating in a terrorist organization and organizing a terrorist act and against three other locals for illegal possession of explosives.[39] ISW is unable to verify the FSB’s claims.
Key Takeaways:
- Multiple reports from Western media indicate that the US government is prohibiting the United Kingdom (UK) from allowing Ukraine to use Storm Shadow missiles to strike military targets in Russia.
- Several Russian milbloggers claimed on August 28 that the tempo of Ukrainian attacks in Kursk Oblast has slowed and that Ukrainian forces are now attempting to dig in and hold select areas they recently seized.
- Ukrainian forces conducted drone strikes against oil infrastructure in Rostov and Kirov oblasts and reportedly conducted a drone strike against Voronezh Oblast overnight on August 27 to 28.
- The prominent Kremlin-linked Rybar Telegram project's continued meetings with Iraqi officials appear to be supporting Iranian-backed Iraqi efforts to gain greater control of their information and media space.
- Kremlin newswire TASS will soon open an office in Iran, supporting Moscow's efforts to deepen its partnership with Tehran.
- Russia's allies appear to be increasingly restricting their transactions with Russian companies and financial institutions amid ongoing concern about the impact of secondary US sanctions.
- The Russian Federal Security Service (FSB) reportedly prevented a terrorist attack in the Republic of Ingushetia on August 28.
- Ukrainian forces recently regained positions in the Siversk direction.
- Russian forces recently advanced southeast of Kupyansk, within Toretsk, southeast of Pokrovsk, and northeast of Vuhledar.
- The Russian Ministry of Defense (MoD) is reportedly supporting the creation of a new type of combat unit that will specialize in using and countering drones, unmanned systems, and other electronic equipment in occupied Zaporizhia Oblast.
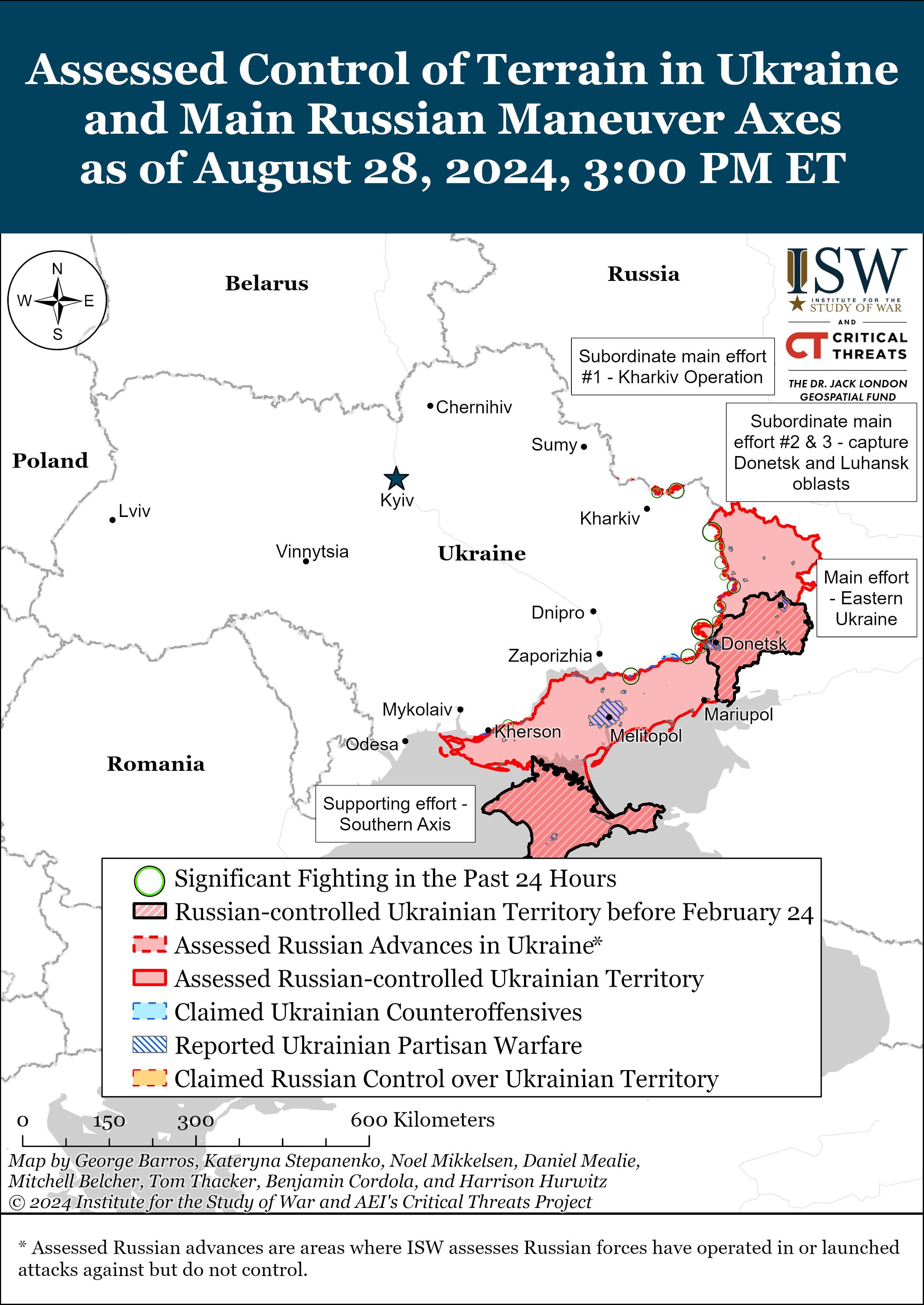
We do not report in detail on Russian war crimes because these activities are well-covered in Western media and do not directly affect the military operations we are assessing and forecasting. We will continue to evaluate and report on the effects of these criminal activities on the Ukrainian military and the Ukrainian population and specifically on combat in Ukrainian urban areas. We utterly condemn Russian violations of the laws of armed conflict and the Geneva Conventions and crimes against humanity even though we do not describe them in these reports.
- Russian Main Effort — Eastern Ukraine (comprised of three subordinate main efforts)
- Russian Subordinate Main Effort #1 — Push Ukrainian forces back from the international border with Belgorod Oblast and approach to within tube artillery range of Kharkiv City
- Russian Subordinate Main Effort #2 — Capture the remainder of Luhansk Oblast and push westward into eastern Kharkiv Oblast and encircle northern Donetsk Oblast
- Russian Subordinate Main Effort #3 — Capture the entirety of Donetsk Oblast
- Russian Supporting Effort — Southern Axis
- Russian Air, Missile, and Drone Campaign
- Russian Mobilization and Force Generation Efforts
- Russian Technological Adaptations
- Activities in Russian-occupied areas
- Ukrainian Defense Industrial Base Efforts
Russian Information Operations and Narratives
- Significant Activity in Belarus
Russian Main Effort — Eastern Ukraine
Russian Subordinate Main Effort #1 — Kharkiv Oblast ( Russian objective: Push Ukrainian forces back from the international border with Belgorod Oblast and approach to within tube artillery range of Kharkiv City)
Russian forces continued offensive operations in northern Kharkiv Oblast on August 28, but there were no confirmed changes the frontline. Russian forces continued offensive operations north of Kharkiv City near Lyptsi and Hlyboke and northeast of Kharkiv City near Vovchansk on August 27 and 28.[40] A Russian milblogger claimed that Ukrainian forces unsuccessfully counterattacked near Lyptsi and Vovchansk.[41] Ukraine's Kharkiv Group of Forces stated that Russian forces are preparing to move elements of the 11th Tank Regiment (18th Motorized Rifle Division, 11th Army Corps [AC], Leningrad Military District [LMD]) to forward positions near Hlyboke and are moving small groups of the 7th Motorized Rifle Regiment (11th AC) near Lukyantsi (north of Kharkiv City) to conduct logistical support and improve Russian communications systems.[42] Ukraine's Kharkiv Group of Forces also stated that elements of the Russian 82nd Motorized Rifle Regiment (69th Motorized Rifle Division, 6th Combined Arms Army [CAA], LMD) are preparing for assault operations in Vovchansk and that elements of the 136th Motorized Rifle Brigade (58th CAA, Southern Military District [SMD]) are conducting a relief in place for elements of the 83rd Airborne (VDV) Brigade near Tykhe (east of Vovchansk). ISW observed reports in early August 2024 that elements of the 136th Motorized Rifle Brigade were operating in western Zaporizhia Oblast.[43] The spokesperson of a Ukrainian brigade operating in the Kharkiv direction stated that Russian forces have very few armored vehicles and are conducting assaults mostly on foot, motorcycles, or buggies.[44] The spokesperson stated that the intensity of Russian assaults in the Kharkiv direction has decreased since the start of the Ukrainian incursion in Kursk Oblast from up to 10 attacks per day to one or two attacks per day.[45]
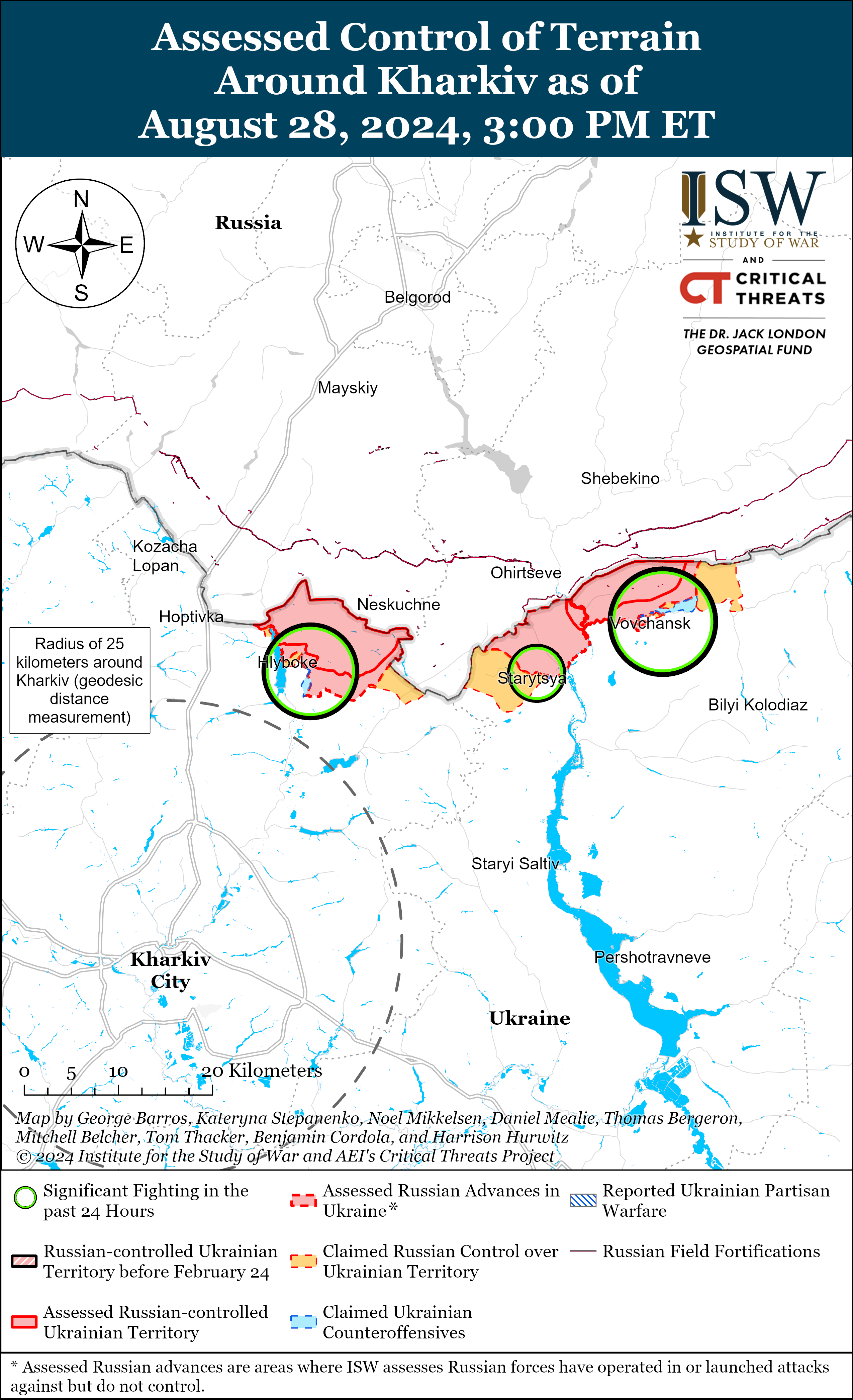
Russian Subordinate Main Effort #2 — Luhansk Oblast (Russian objective: Capture the remainder of Luhansk Oblast and push westward into eastern Kharkiv Oblast and northern Donetsk Oblast)
Russian forces recently advanced southeast of Kupyansk amid continued offensive operations along the Kupyansk-Svatove-Kreminna line on August 28. Geolocated footage published on August 28 indicates that Russian forces advanced northwest of Pishchane (southeast of Kupyansk).[46] Russian forces continued attacking northeast of Kupyansk near Synkivka; southeast of Kupyansk near Stelmakhivka, Stepova Novoselivka, Kolisnykivka, and Kruhlyakivka; southwest of Svatove near Druzhelyubivka; northwest of Kreminna near Novosadove, Nevske, and Makiivka; and west of Kreminna near Terny on August 27 and 28.[47]
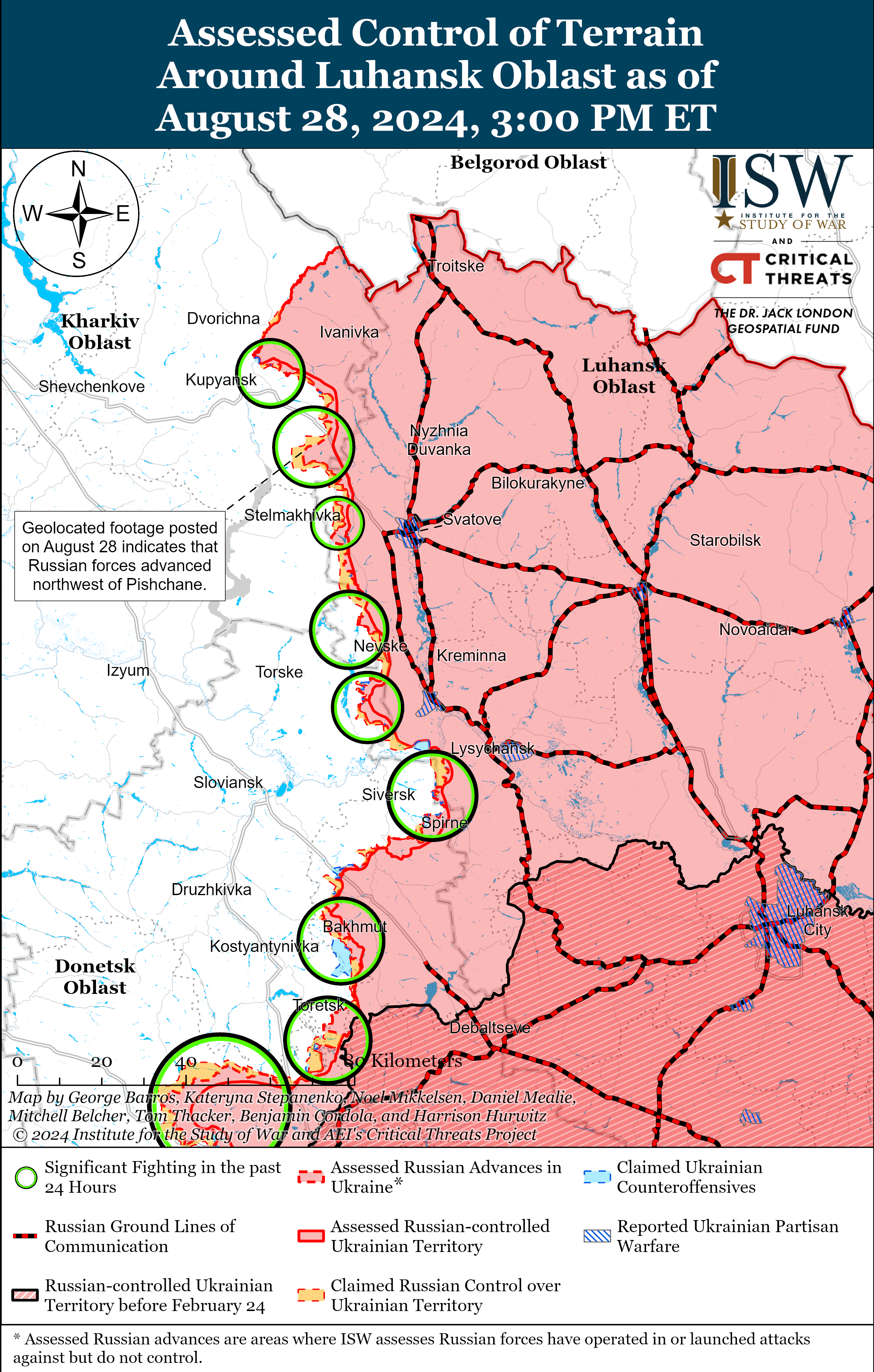
Russian Subordinate Main Effort #3 — Donetsk Oblast (Russian objective: Capture the entirety of Donetsk Oblast, the claimed territory of Russia’s proxies in Donbas)
Ukrainian forces recently regained lost positions in the Siversk direction amid continued Russian offensive operations in the area on August 28. Geolocated footage published on August 27 indicates that Ukrainian forces recently regained lost positions southwest of Ivano-Darivka (southeast of Siversk).[48] Russian forces attacked west of Siversk near Verkhnokamyanske and southeast of Siversk near Vyimka, Ivano-Darivka, and Spirne on August 27 and 28.[49] A Russian battalion commander from the 123rd Motorized Rifle Brigade (2nd Luhansk People's Republic Army Corps [LNR AC]) operating in the Siversk direction told Kremlin newswire TASS in an August 28 article that Russian forces in the area first conduct artillery and first-person view (FPV) drone strikes against Ukrainian positions and then conduct ground attacks in small infantry groups.[50]
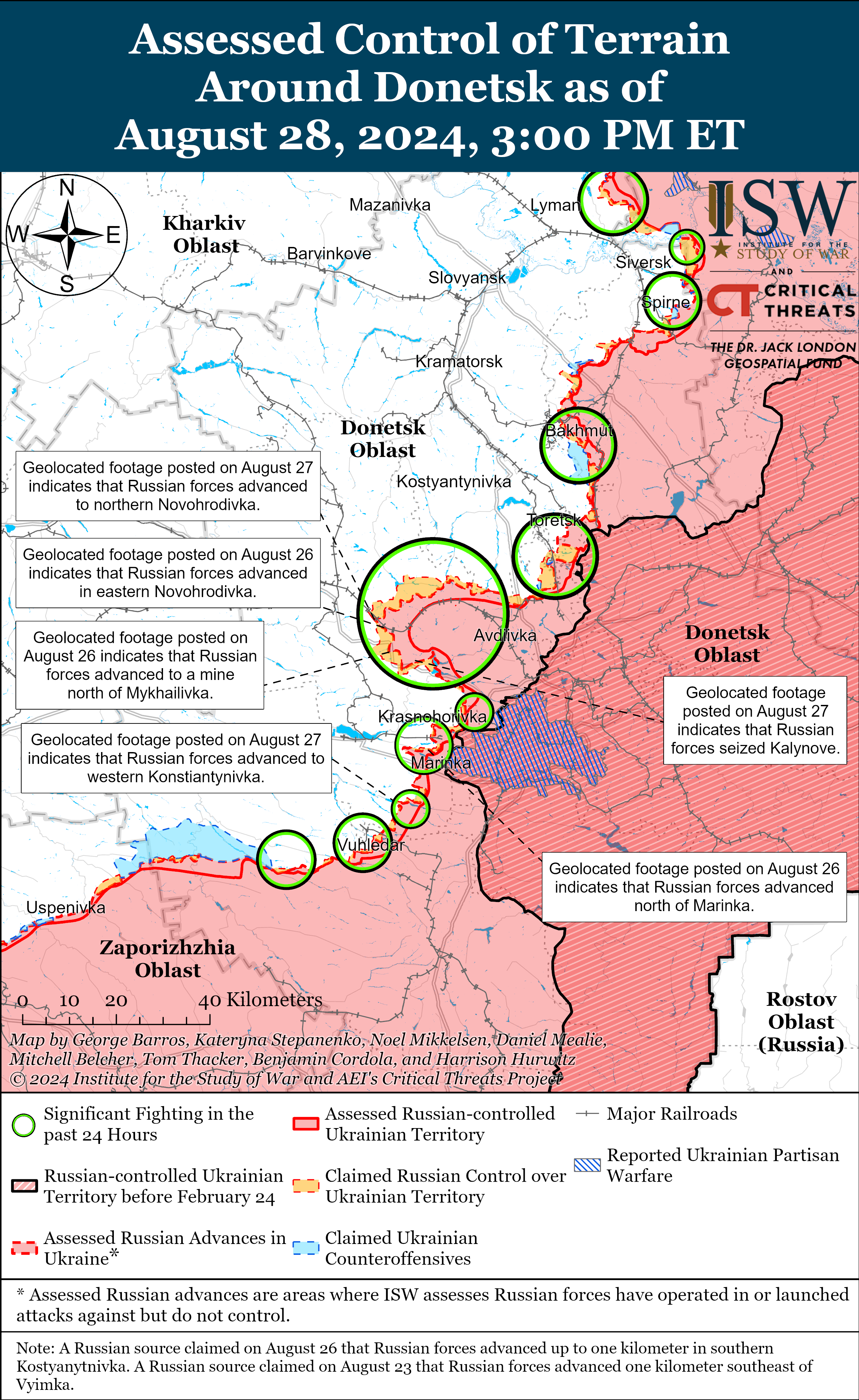
Russian forces continued offensive operations in the Chasiv Yar direction on August 28 but did not make any confirmed advances. Russian state news outlet RIA Novosti claimed as of August 28 that Russian forces advanced west of Andriivka and Kurdyumivka (both southeast of Chasiv Yar).[51] Russian forces attacked within eastern Chasiv Yar; north of Chasiv Yar near Hryhorivka; southeast of Chasiv Yar near Ivanivske, Klishchiivka, and Andriivka; and south of Chasiv Yar near Bila Hora on August 27 and 28.[52]
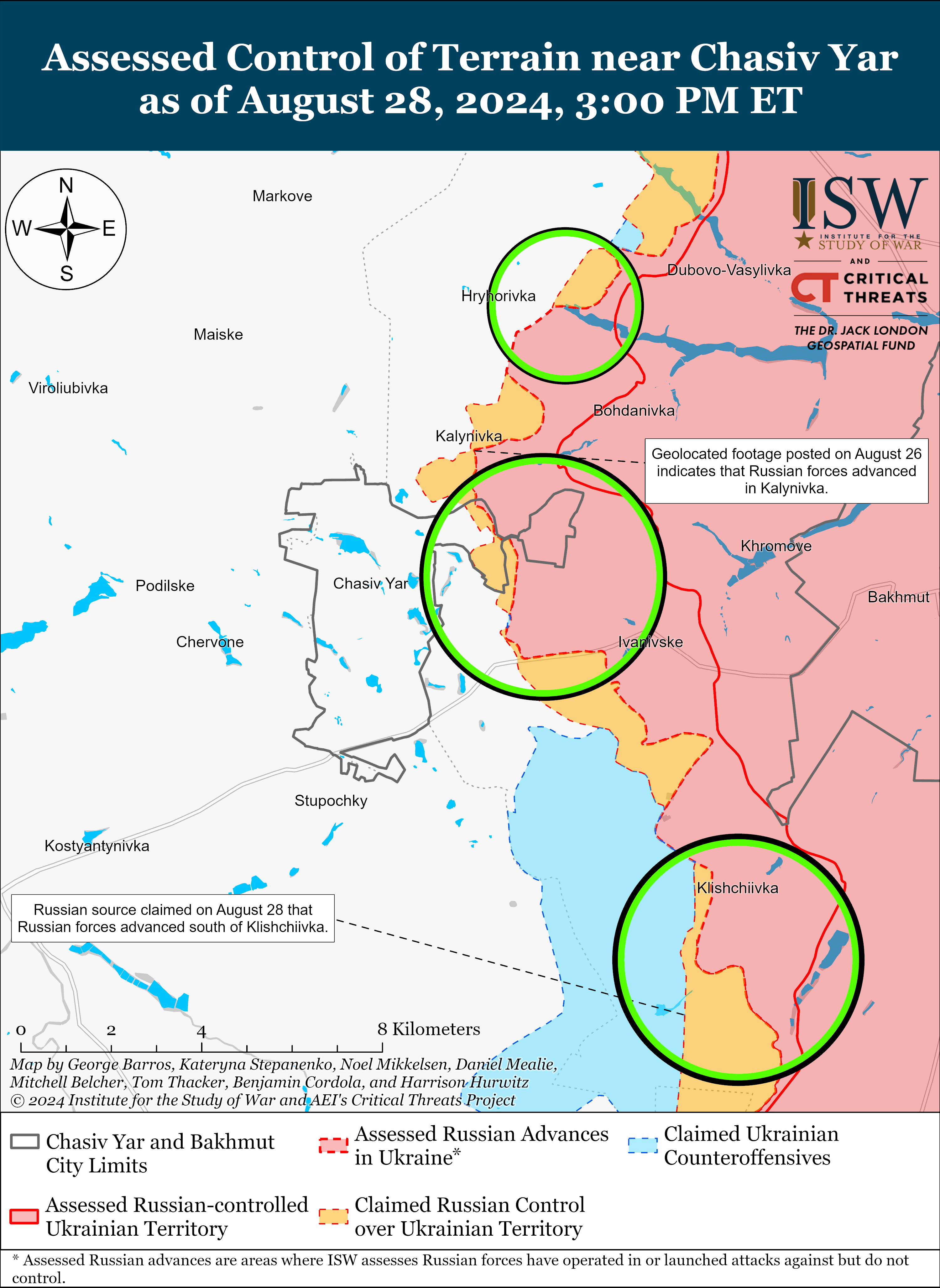
Ukrainian military officials reported that Ukrainian forces shot down a Russian Su-35 fighter jet with man-portable air-defense systems (MANPADS) near Ozaryanivka (southeast of Chasiv Yar) on August 28.[53] A representative of the Ukrainian brigade that downed the Russian Su-35 stated that the brigade is unable to confirm the status of the Russian crew or sufficiently conduct a battle damage assessment since the aircraft fell into Russian-occupied Ukraine.[54]
Russian forces recently advanced within Toretsk amid continued Russian offensive operations in the area on August 28. Geolocated footage published on August 28 indicates that Russian forces recently advanced within eastern Toretsk along 91 Dyvizyi Street.[55] Russian forces continued attacking near Toretsk; east of Toretsk near Druzhba; southeast of Toretsk near Pivnichne; and south of Toretsk near Niu York and Nelipivka.[56]
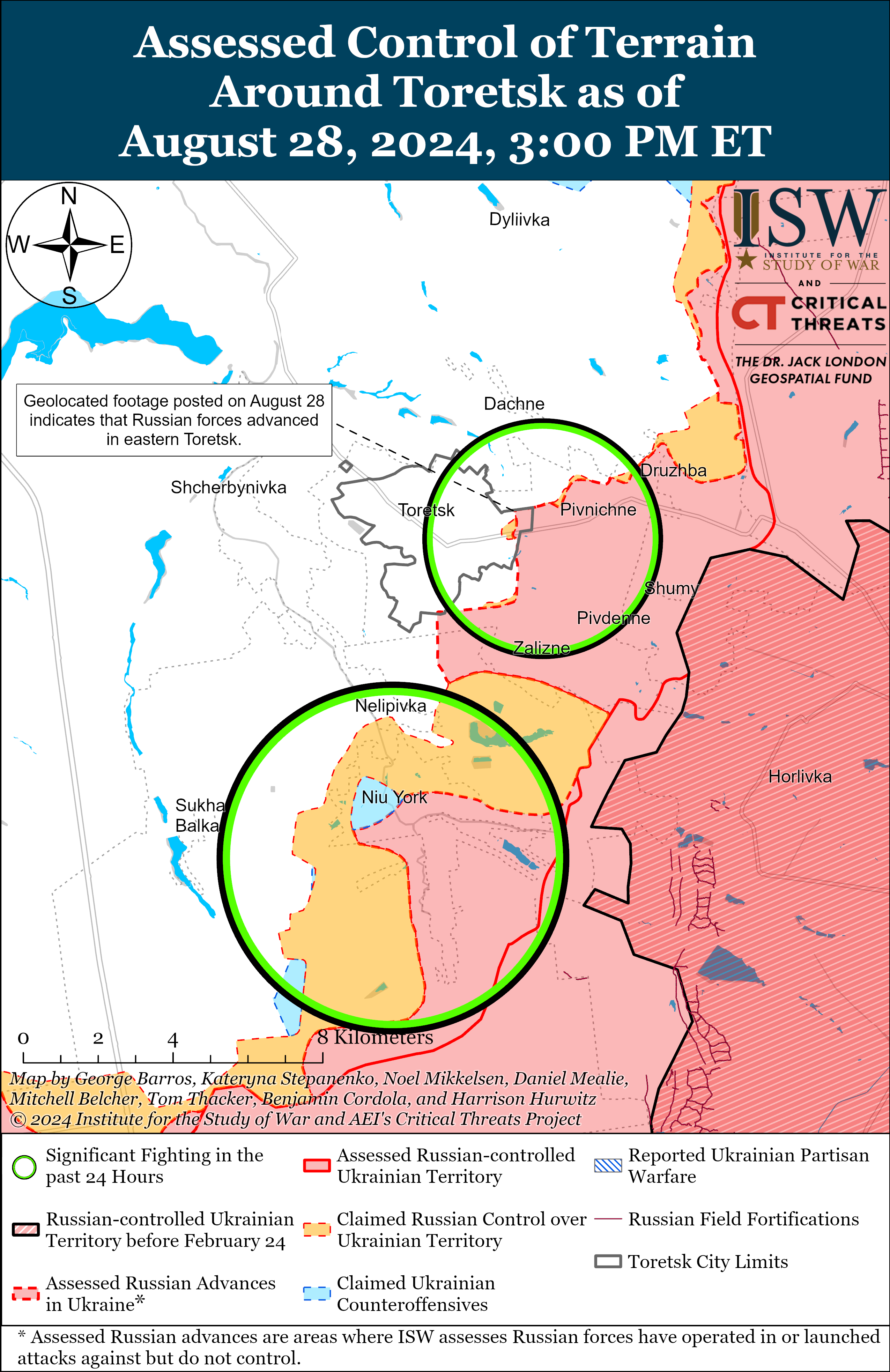
Russian forces continue to make significant tactical advances southeast of Pokrovsk. Geolocated footage published on August 28 indicates that Russian forces have advanced into central Memryk (southeast of Pokrovsk) as Russian milbloggers continued to claim that Russian forces have completely seized the settlement.[57] Additional geolocated footage published on August 28 indicates that Russian forces have advanced southeast of Krasnyi Yar (also southeast of Pokrovsk) along Artema (Vyshneva) Street, which runs from Krasnyi Yar into Hrodivka (east of Pokrovsk).[58] Russian milbloggers claimed that Russian forces have also advanced up to the Zhuravka River in central Hrodivka and reached the southern outskirts of Myrnohrad (due east of Pokrovsk) via Krasnyi Yar and Mykolaivka.[59] Russian milbloggers continued to claim that Russian forces are advancing in and near Novohrodivka (southeast of Pokrovsk), and Ukrainian military observer Kostyantyn Mashovets noted that elements of the 27th Motorized Rifle Division (2nd Combined Arms Army [CAA], Central Military District [CMD]) with support from the 114th Motorized Rifle Brigade (1st Donetsk People's Republic Army Corps [DNR AC]) are pushing along the railway in eastern Novohrodivka.[60] Russian and some Ukrainian sources reported that Russian forces have reached the eastern outskirts of Selydove (further southeast of Pokrovsk) and are advancing into the town following Ukrainian withdrawals from some positions in eastern Selydove.[61] ISW has not yet observed visual confirmation of Russian forces operating in Selydove, but Mashovets reported on August 28 that elements of the 90th Tank Division (41st CAA, CMD), with support of elements of the 27th Motorized Rifle Division managed to break through towards Selydove from the north along the E50 Selydove-Karlivka route, which is consistent with recent Russian claims of Russian advances into Selydove via Mykhailivka (just east of Selydove).[62] The Ukrainian General Staff reported a high rate of Russian attacks northeast, east, and southeast of Pokrovsk on August 27 and 28, and noted that over half of the Russian ground attacks in the Pokrovsk direction on August 28 were concentrated near Selydove and Novohrodivka.[63] Russian Defense Minister Andrei Belousov congratulated the Russian 589th and 433rd motorized rifle regiments (27th Motorized Rifle Division, 2nd CAA, CMD) for their recent gains southeast of Pokrovsk.[64] Elements of the Russian "White Wolves" Battalion reportedly recently seized Komyshivka (about 20km southeast of Pokrovsk).[65]
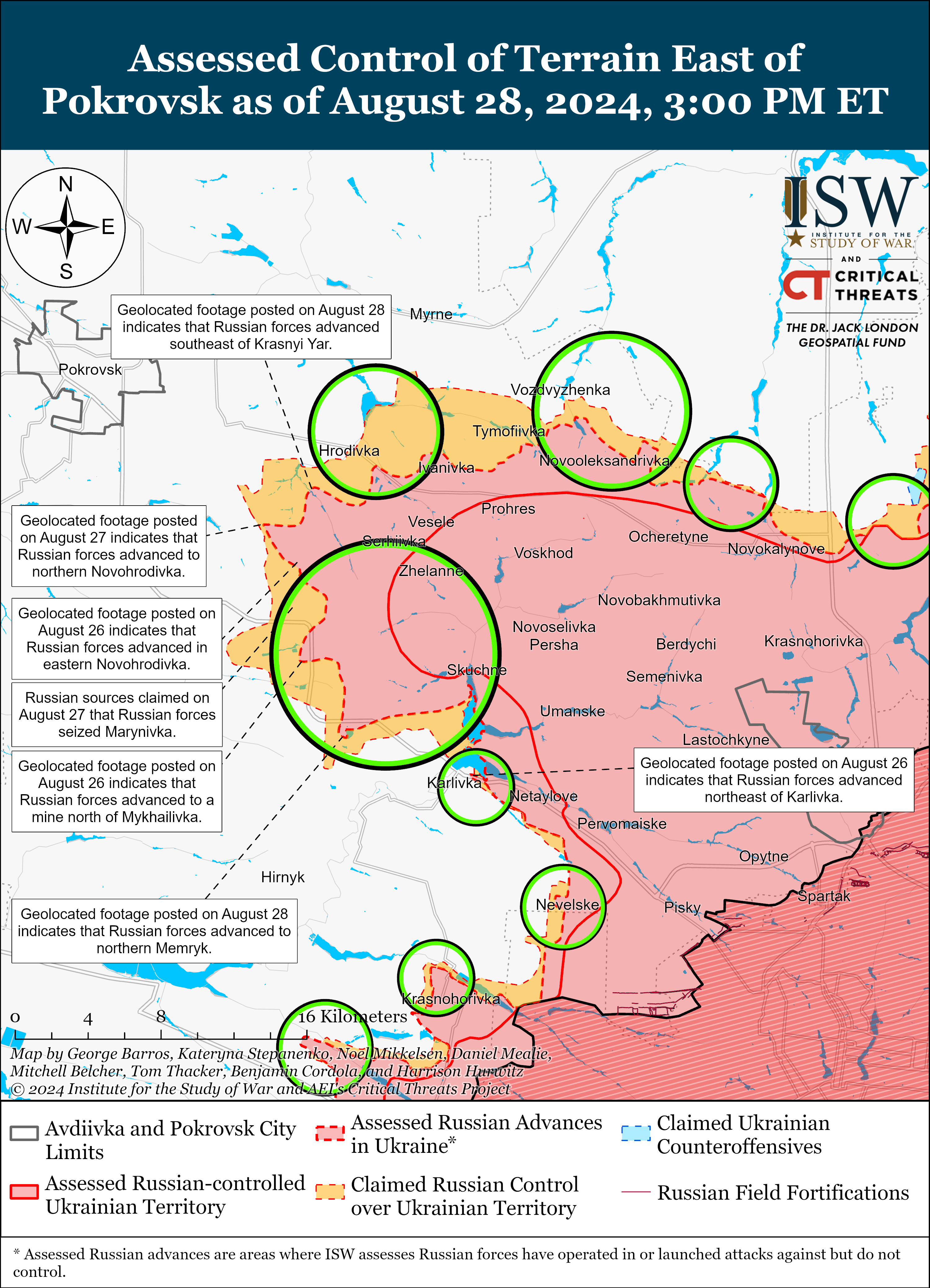
Russian forces continued offensive operations west of Donetsk City on August 28 but did not make any confirmed advances. The Ukrainian General Staff reported that Russian forces continued ground attacks near Krasnohorivka and Heorhiivka (both west of Donetsk City) on August 27 and 28.[66]
Russian forces recently advanced in the Vuhledar direction (southwest of Donetsk City) amid continued offensive operations along the T05024 Marinka-Vuhledar highway on August 28. Geolocated footage published on August 27 shows Russian forces, reportedly of the Republic of Tatarstan volunteer-based 1st "Timer" Battalion of the 57th Motorized Rifle Regiment (6th Motorized Rifle Division, 3rd AC), planting a flag in western Kostyantynivka (northeast of Vuhledar) at a position along Shakhtarska Street, indicating that Russian forces have likely seized the whole settlement.[67] Several Russian milbloggers claimed that Russian forces have completely seized Kostyantynivka, although one Russian milblogger noted that reports of Kostyantynivka's capture may be premature because some of the settlement remains a contested grey zone.[68] A prominent Kremlin-affiliated milblogger claimed that Russian forces have also crossed the T05024 road southwest of Vodyane (just northeast of Vuhledar), although ISW has not yet seen visual evidence of this claim.[69] Unspecified Russian security forces told Kremlin newswire TASS on August 28 that Russian forces have interdicted two main ground lines of communication (GLOCS) into Vuhledar, likely in reference to the T05024 and T0509 Vuhledar-Velyka Novosilka road, both of which geolocated footage confirms that Russian forces have crossed in limited areas.[70] The Ukrainian General Staff reported Russian ground attacks northeast of Vuhledar near Kostyantynivka and Vodyane; near Vuhledar itself; and west of Vuhledar near Prechystivka.[71]
Neither Russian nor Ukrainian sources reported offensive operations in the Donetsk-Zaporizhia Oblast border area near Velyka Novosilka on August 28.
Russian Supporting Effort — Southern Axis (Russian objective: Maintain frontline positions and secure rear areas against Ukrainian strikes)
Positional engagements continued in western Zaporizhia Oblast near Robotyne, Novodanylivka (north of Robotyne), and Mala Tokmachka (northeast of Robotyne) on August 27 and 28.[72] Elements of the Russian "Gnom" drone detachment are reportedly operating in the Zaporizhia direction.[73]
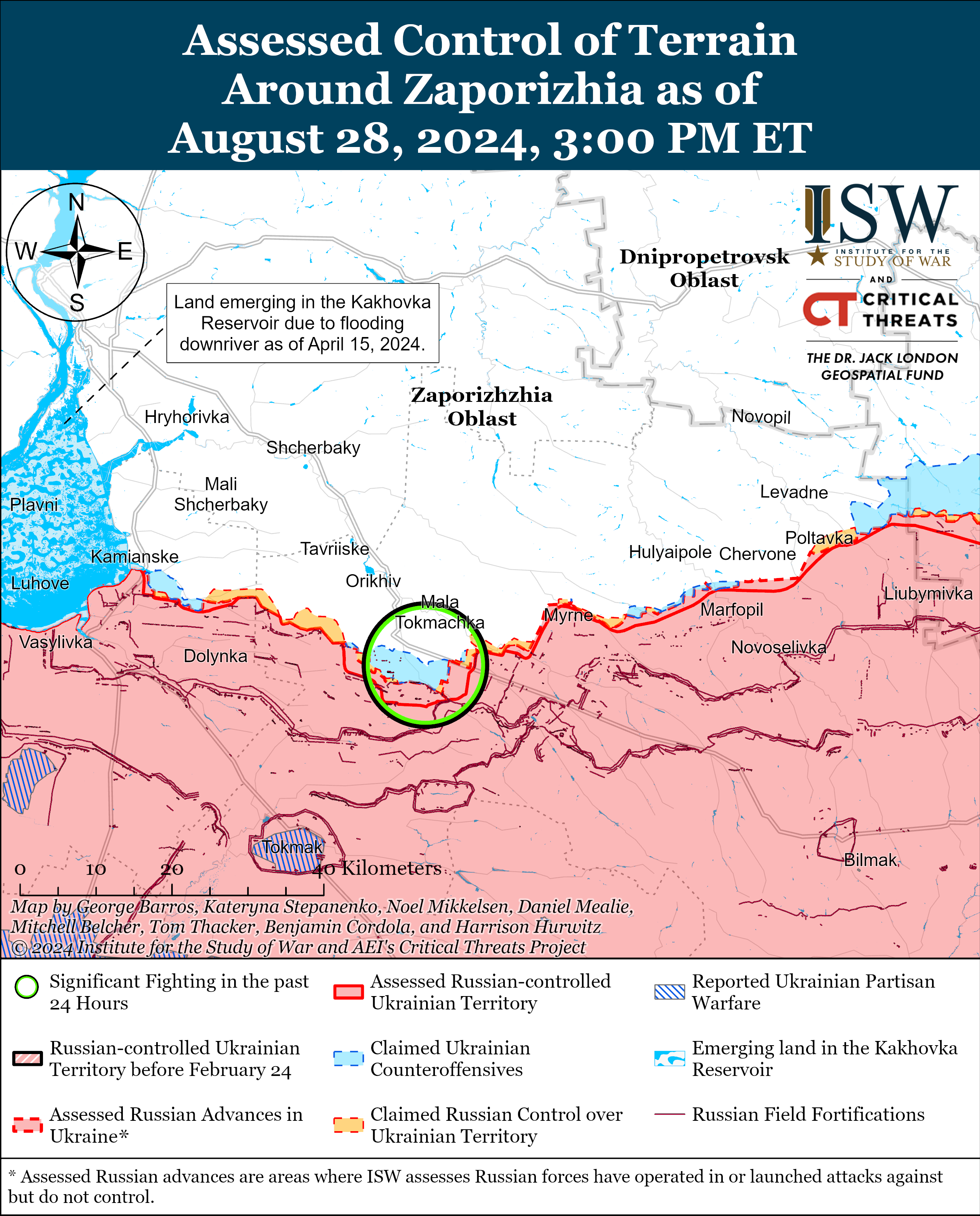
Positional engagements continued in the Kherson direction on August 27 and 28.[74]
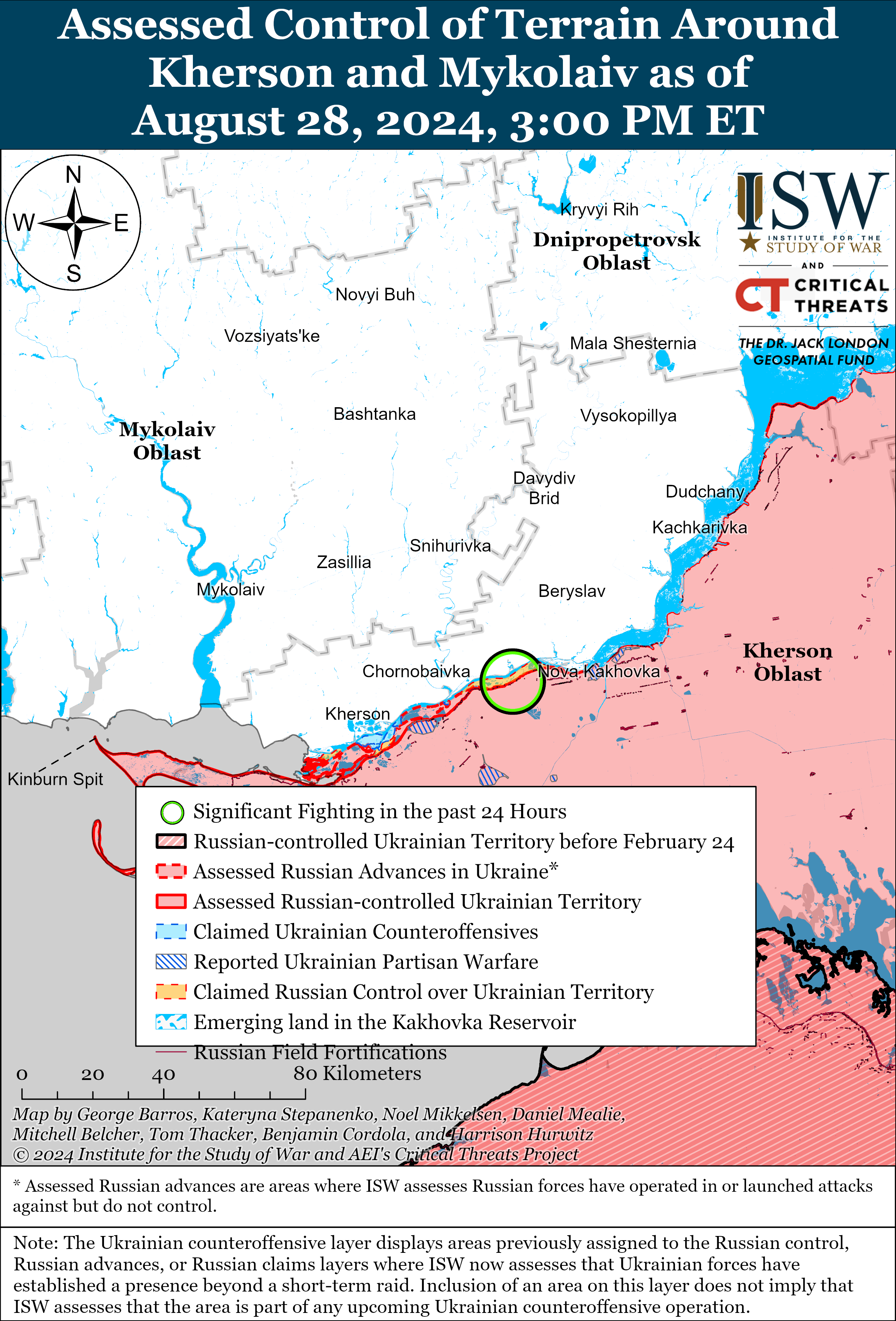
Russian Air, Missile, and Drone Campaign (Russian Objective: Target Ukrainian military and civilian infrastructure in the rear and on the frontline)
Russian forces conducted limited missile strikes against Ukraine on the night of August 27 and the morning of August 28. The Ukrainian Ministry of Internal Affairs reported that Russian forces launched two Kh-59 cruise missiles strikes against Izyum, Kharkiv Oblast on the night of August 27.[75] Dnipropetrovsk Oblast Military Administration Head Serhii Lysak reported that Russian forces struck Kryvyi Rih with an unspecified munition on the morning of August 28, injuring nine civilians.[76]
Russian Mobilization and Force Generation Efforts (Russian objective: Expand combat power without conducting general mobilization)
The Russian Ministry of Defense (MoD) is reportedly supporting the creation of a new type of combat unit that will specialize in using and countering drones, unmanned systems, and other electronic equipment in occupied Zaporizhia Oblast. Former Roscosmos (Russian space agency) head and Zaporizhia Oblast occupation senator Dmitry Rogozin announced on August 28 that the Russian MoD is sponsoring the creation of the "BARS-SARMAT" Detachment, which Rogozin characterized as a volunteer detachment specializing in robotic electronic complexes and systems.[77] Rogozin encouraged his subscribers to amplify this news and stated that the detachment will test and use new drones, robotic systems, electronic reconnaissance and combat systems, counter-battery systems, and control and communications equipment in combat conditions. Rogozin stated that the detachment wants to recruit software developers, cybersecurity specialists, technicians, drone manufacturers and designers, and other specialists to support the detachment's work. Rogozin noted that the BARS-SARMAT Detachment also wants to recruit women as well as students from technical universities who are willing to take academic leave. Rogozin supervises several BARS units, including the Russian BARS-10 Battalion of the "Tsarskiye Volki" Brigade, and has recently advocated for the Russian MoD to encourage greater competition and variety among Russian technological innovation and drone production initiatives.[78] Several Russian sources amplified Rogozin's announcement on August 28 and noted that the "BARS-SARMAT" Detachment is forming in occupied Zaporizhia Oblast and will provide feedback on the battlefield utility of new technology to the Russian MoD and Russian weapons developers.[79]
Russian Technological Adaptations (Russian objective: Introduce technological innovations to optimize systems for use in Ukraine)
A Russian milblogger insinuated that Russian forces are attempting to bypass Ukrainian electronic warfare (EW) systems by flying their drones at higher altitudes. The milblogger amplified footage on August 28 purportedly showing a Russian drone operator flying a drone at an altitude of roughly 107-108 meters before diving the drone to strike a nearby Ukrainian artillery system.[80] The milblogger claimed that the drone operator was able to overcome Ukrainian EW interference by operating the drone at a high altitude and then quickly causing the drone to plummet and strike the Ukrainian system.
Russian milbloggers expressed concern on August 28 that recent Ukrainian efforts to target Russian reconnaissance drones with first-person view (FPV) drones will impact Russia's reconnaissance and strike capabilities in Ukraine.[81]
Ukrainian Defense Industrial Efforts (Ukrainian objective: Develop its defense industrial base to become more self-sufficient in cooperation with US, European, and international partners)
ISW is suspending publishing coverage of Ukrainian defense industrial efforts until further notice.
Activities in Russian-occupied areas (Russian objective: Consolidate administrative control of annexed areas; forcibly integrate Ukrainian citizens into Russian sociocultural, economic, military, and governance systems)
ISW is not publishing coverage of activities in Russian-occupied areas today.
Russian officials continued to blame Ukraine for threatening nuclear security in Kursk Oblast, including by misrepresenting recent statements by International Atomic Energy Agency (IAEA) Director General Rafael Mariano Grossi's after his August 27 visit to the Kursk Nuclear Power Plant (KNPP).[82] Ukraine's Kharkiv Group of Forces stated that Ukraine does not pose a threat to the KNPP and that Ukrainian forces are taking a responsible approach to nuclear and radiation safety.[83]
Ukraine's Kharkiv Group of Forces responded to Russian Foreign Minister Sergei Lavrov's August 27 claim that Russia is reviewing its nuclear doctrine, noting that multiple high-ranking Russian officials, including Russian President Vladimir Putin, have voiced similar claims in recent months.[84] The Kharkiv Group of Forces stated that these Russian claims are meant to intimidate the West so that the West continues to restrict Ukraine's ability to strike military targets within Russia with Western-provided weapons.
Significant activity in Belarus (Russian efforts to increase its military presence in Belarus and further integrate Belarus into Russian-favorable frameworks and Wagner Group activity in Belarus)
Russian Ministry of Foreign Affairs (MFA) Spokesperson Maria Zakharova claimed on August 28 that Ukrainian calls for Belarus to withdraw forces from the international border area are "unacceptable" and that Belarus is simply fulfilling its obligations to help Russia defend itself from foreign attacks.[85] Ukraine's State Border Service Spokesperson Andriy Demchenko reported on August 27 that Belarusian forces are likely building up near the Ukrainian border in order to stretch and divert Ukrainian forces, consistent with ISW's August 26 assessment about the intentions of the reported Belarusian build-up.[86] ISW continues to assess that it is very unlikely that Belarus will invade Ukraine or enter the war on Russia's behalf.[87]
Note: ISW does not receive any classified material from any source, uses only publicly available information, and draws extensively on Russian, Ukrainian, and Western reporting and social media as well as commercially available satellite imagery and other geospatial data as the basis for these reports. References to all sources used are provided in the endnotes of each update.

[1] https://www.ft.com/content/274d8994-c0fb-4f9b-8572-0ffb00b5de23
[2] https://www.telegraph.co.uk/news/2024/08/27/uk-backs-ukraine-use-storm-shadow-missiles-russia/; https://archive dot ph/VQaIf
[3] https://isw.pub/UkrWar082424; https://www.understandingwar.org/backgrounder/interactive-map-hundreds-known-russian-military-objects-are-range-atacms
[4] https://t.me/smotri_z/34020; https://t.me/sashakots/48661 ; https://t.me/rusich_army/16823 ; https://t.me/dva_majors/50878
[5] https://t.me/sashakots/48660; https://t.me/epoddubny/20879 ; https://t.me/DnevnikDesantnika/14650
[6] https://t.me/black_swan_ukraine/183; https://x.com/EjShahid/status/1828803134417957344; https://x.com/EjShahid/status/1828803389154803745
[7] https://t.me/z_arhiv/27835
[8] https://t.me/rusich_army/16823 ; https://t.me/rybar/63113 ; https://t.me/dva_majors/50878
[9] https://t.me/mod_russia/42686 ; https://t.me/dva_majors/50878 ; https://t.me/rusich_army/16823 ; https://t.me/wargonzo/21790
[10] https://t.me/dva_majors/50856
[11] https://t.me/tass_agency/268942
[12] https://isw.pub/UkrWar082524 ; https://isw.pub/UkrWar082424 ; https://isw.pub/UkrWar082524 ; https://isw.pub/UkrWar081924
[13] https://t.me/IvanovoNovosti/25483 ; https://t.me/horizontal_russia/39454 ; https://t.me/astrapress/62952
[14] https://t.me/horizontal_russia/39454 ; https://t.me/IvanovoNovosti/25483 ; https://t.me/astrapress/62952
[15] https://t.me/mobilizationnews/19990
[16] https://suspilne dot media/822993-ekstrenne-zasidanna-radi-ukraina-nato-ukraina-stvorila-vlasnu-balisticnu-raketu-917-den-vijni-onlajn/?anchor=live_1724834944&utm_source=copylink&utm_medium=ps
[17] https://t.me/golubev_vu/1507
[18] https://t.me/rybar/63112
[19] https://suspilne dot media/823573-gur-mo-vperse-vrazilo-dronami-naftobazu-u-kirovskij-oblasti-rf-dzerela/
[20] https://t.me/tass_agency/268952
[21] https://ria dot ru/20240828/kotelnich-1968954199.html
[22] https://t.me/gusev_36/2692
[23] https://t.me/rybar/63112
[24] https://t.me/mod_russia/42668
[25] https://t.me/dva_majors/50885; https://t.me/tass_agency/268966
[26] https://t.me/rybar/63111
[27] https://www.understandingwar.org/backgrounder/leadership-and-purpose-iraq%E2%80%99s-popular-mobilization-forces
[28] https://www.washingtoninstitute.org/policy-analysis/iraqs-resistance-factions-shift-major-crackdown-media-spaces; https://www.washingtoninstitute.org/policy-analysis/iraqi-cmc-draft-regulation-digital-content-iraq
[29] https://isw.pub/UkrWar073024; https://isw.pub/UkrWar071224; https://isw.pub/UkrWar061124
[30] https://t.me/tass_agency/268855
[31] https://rg dot ru/2024/08/28/novosti-so-znakom-kachestva.html; https://tass dot ru/obschestvo/21703425
[32] https://www.understandingwar.org/backgrounder/russian-offensive-campaign-assessment-june-26-2024; https://isw.pub/UkrWar070624; https://isw.pub/UkrWar070924; https://isw.pub/UkrWar071324
[33] https://www.kommersant dot ru/doc/6919850?from=top_main_3
[34] https://www.forbes dot ru/finansy/519570-perevody-cerez-rossijskie-banki-prekratili-osusestvlat-12-bankov-kirgizii ; https://24 dot kg/ekonomika/302630_sanktsii_udarili_poperevodam_kakie_bankikr_vse_esche_otpravlyayut_dengi_vrossiyu_/ ; https://www.vedomosti dot ru/finance/news/2024/08/19/1056584-kirgizskii-mbank ; https://t.me/tass_agency/267816 ; https://m.business-gazeta dot ru/news/645383 ;
[35] https://24 dot kg/ekonomika/302630_sanktsii_udarili_poperevodam_kakie_bankikr_vse_esche_otpravlyayut_dengi_vrossiyu_/
[36] https://ofac.treasury.gov/recent-actions/20240823
[37] https://t.me/tass_agency/268913; https://t.me/tass_agency/268906; https://t.me/tass_agency/268903
[38] https://t.me/tass_agency/268909
[39] https://ingushetia.sledcom dot ru/news/item/1909904/
[40] https://www.facebook.com/GeneralStaff.ua/posts/pfbid02hvYvDy7aPcowjP6Tsya7omtQuMduZK4BxRneMhrCoeGS3QAt7hqV1imtW95tbpMhl ; https://www.facebook.com/GeneralStaff.ua/posts/pfbid0ZQNnW7qYx5knBHTJJEMhKw5Pv7uasPG8q3kCjpbFVJCyBTcvZbRe1rmXEpKtL8Sql ; https://www.facebook.com/GeneralStaff.ua/posts/pfbid02qUUyqRxjK1MKSTh5hjknbf7xGDXZtrB7U2H3AL28KceNo6rYyFVjy4RaN3ia8QU9l; https://t.me/wargonzo/21791
[41] https://t.me/Sladkov_plus/11273
[42] https://t.me/otukharkiv/941
[43] https://www.understandingwar.org/backgrounder/russian-offensive-campaign-assessment-august-5-2024
[44] https://armyinform.com dot ua/2024/08/28/u-dshv-zsu-rozpovily-pro-zminu-taktyky-protyvnyka-na-harkivshhyni/
[45] https://armyinform dot com.ua/2024/08/28/u-dshv-zsu-rozpovily-pro-zminu-taktyky-protyvnyka-na-harkivshhyni/ ; https://www.youtube.com/watch?v=j5TqaWCCdUg
[46] https://t.me/creamy_caprice/6565; https://www.facebook.com/RDTerritorialDefenseForcesNorth/videos/544194034604539/?mibextid=rS40aB7S9Ucbxw6v
[47] https://www.facebook.com/GeneralStaff.ua/posts/pfbid02hvYvDy7aPcowjP6Tsya7omtQuMduZK4BxRneMhrCoeGS3QAt7hqV1imtW95tbpMhl ; https://www.facebook.com/GeneralStaff.ua/posts/pfbid0ZQNnW7qYx5knBHTJJEMhKw5Pv7uasPG8q3kCjpbFVJCyBTcvZbRe1rmXEpKtL8Sql ; https://www.facebook.com/GeneralStaff.ua/posts/pfbid02qUUyqRxjK1MKSTh5hjknbf7xGDXZtrB7U2H3AL28KceNo6rYyFVjy4RaN3ia8QU9l
[48] https://x.com/AudaxonX/status/1828734164188713195; https://t.me/BattalionSIGIRIYA/87
[49] https://www.facebook.com/GeneralStaff.ua/posts/pfbid02hvYvDy7aPcowjP6Tsya7omtQuMduZK4BxRneMhrCoeGS3QAt7hqV1imtW95tbpMhl ; https://www.facebook.com/GeneralStaff.ua/posts/pfbid0ZQNnW7qYx5knBHTJJEMhKw5Pv7uasPG8q3kCjpbFVJCyBTcvZbRe1rmXEpKtL8Sql ; https://www.facebook.com/GeneralStaff.ua/posts/pfbid02qUUyqRxjK1MKSTh5hjknbf7xGDXZtrB7U2H3AL28KceNo6rYyFVjy4RaN3ia8QU9l
[50] https://tass dot ru/armiya-i-opk/21704051
[51] https://ria dot ru/20240828/spetsoperatsiya-1968946235.html
[52] https://www.facebook.com/GeneralStaff.ua/posts/pfbid02hvYvDy7aPcowjP6Tsya7omtQuMduZK4BxRneMhrCoeGS3QAt7hqV1imtW95tbpMhl ; https://www.facebook.com/GeneralStaff.ua/posts/pfbid0ZQNnW7qYx5knBHTJJEMhKw5Pv7uasPG8q3kCjpbFVJCyBTcvZbRe1rmXEpKtL8Sql ; https://www.facebook.com/GeneralStaff.ua/posts/pfbid02qUUyqRxjK1MKSTh5hjknbf7xGDXZtrB7U2H3AL28KceNo6rYyFVjy4RaN3ia8QU9l ; https://t.me/DnevnikDesantnika/14635 ; https://t.me/wargonzo/21791
[53] https://t.me/Pavliuk_KSV/5569 ; https://t.me/Khortytsky_wind/980 ; https://armyinform.com dot ua/2024/08/28/lyczari-rozkryly-podrobyczi-zbyttya-rosijskogo-su-25/
[54] https://armyinform.com dot ua/2024/08/28/lyczari-rozkryly-podrobyczi-zbyttya-rosijskogo-su-25/
[55] https://t.me/skalabatalion/246; https://x.com/MikiValbuena/status/1828863805620695520
[56] https://www.facebook.com/GeneralStaff.ua/posts/pfbid02hvYvDy7aPcowjP6Tsya7omtQuMduZK4BxRneMhrCoeGS3QAt7hqV1imtW95tbpMhl ; https://www.facebook.com/GeneralStaff.ua/posts/pfbid0ZQNnW7qYx5knBHTJJEMhKw5Pv7uasPG8q3kCjpbFVJCyBTcvZbRe1rmXEpKtL8Sql ; https://www.facebook.com/GeneralStaff.ua/posts/pfbid02qUUyqRxjK1MKSTh5hjknbf7xGDXZtrB7U2H3AL28KceNo6rYyFVjy4RaN3ia8QU9l ; https://t.me/wargonzo/21791
[57] https://x.com/AudaxonX/status/1828729455960809718; https://t.me/warhistoryalconafter/181731; https://t.me/dva_majors/50856; https://t.me/DnevnikDesantnika/14656; https://t.me/wargonzo/21791; https://t.me/RVvoenkor/75827; https://t.me/RVvoenkor/75837 ; https://t.me/boris_rozhin/135227 ; https://t.me/SolovievLive/276743 ; https://t.me/milinfolive/129399; https://t.me/notes_veterans/19153
[58] https://x.com/NOELreports/status/1828735672586219550; https://x.com/vosintt/status/1828741998884409714; https://www.facebook.com/71brigade/videos/499752789440715/?mibextid=jmPrMh&rdid=m3nShiGF0hDjtjGQ; https://t.me/creamy_caprice/6562
[59] https://t.me/voenkorKotenok/58605; https://t.me/motopatriot/26829; https://t.me/voenkorKotenok/58609; https://t.me/boris_rozhin/135242; https://t.me/z_arhiv/27829; https://t.me/z_arhiv/27824
[60] https://t.me/zvizdecmanhustu/2144
[61] https://t.me/tass_agency/268925; https://t.me/RVvoenkor/75794; https://t.me/RVvoenkor/75827; https://t.me/dva_majors/50848; https://t.me/RVvoenkor/75839 ; https://t.me/boris_rozhin/135227; https://t.me/z_arhiv/27824; https://t.me/z_arhiv/27839; https://t.me/boris_rozhin/135176; https://t.me/voenkorKotenok/58597; https://t.me/boris_rozhin/135242
[62] https://t.me/zvizdecmanhustu/2144
[63] https://www.facebook.com/GeneralStaff.ua/posts/pfbid02hvYvDy7aPcowjP6Tsya7omtQuMduZK4BxRneMhrCoeGS3QAt7hqV1imtW95tbpMhl; https://www.facebook.com/GeneralStaff.ua/posts/pfbid0ZQNnW7qYx5knBHTJJEMhKw5Pv7uasPG8q3kCjpbFVJCyBTcvZbRe1rmXEpKtL8Sql; https://www.facebook.com/GeneralStaff.ua/posts/pfbid02qUUyqRxjK1MKSTh5hjknbf7xGDXZtrB7U2H3AL28KceNo6rYyFVjy4RaN3ia8QU9l
[64] https://t.me/mod_russia/42664
[65] https://t.me/RVvoenkor/75834 ; https://t.me/btr80/19894 ; https://t.me/boris_rozhin/135245
[66] https://www.facebook.com/GeneralStaff.ua/posts/pfbid02hvYvDy7aPcowjP6Tsya7omtQuMduZK4BxRneMhrCoeGS3QAt7hqV1imtW95tbpMhl; https://www.facebook.com/GeneralStaff.ua/posts/pfbid0ZQNnW7qYx5knBHTJJEMhKw5Pv7uasPG8q3kCjpbFVJCyBTcvZbRe1rmXEpKtL8Sql; https://www.facebook.com/GeneralStaff.ua/posts/pfbid02qUUyqRxjK1MKSTh5hjknbf7xGDXZtrB7U2H3AL28KceNo6rYyFVjy4RaN3ia8QU9l
[67] https://t.me/krylataya_pehota_z/1877; https://t.me/creamy_caprice/6559; ; https://t.me/voin_dv/10499
[68] https://t.me/krylataya_pehota_z/1877; https://t.me/creamy_caprice/6559; https://t.me/rybar/63104; https://t.me/NgP_raZVedka/18858; https://t.me/DnevnikDesantnika/14632; https://t.me/z_arhiv/27821; https://t.me/RVvoenkor/75805; https://x.com/JulianRoepcke/status/1828753966454472744; https://t.me/motopatriot/26838; https://t.me/motopatriot/26839
[69] https://t.me/rybar/63104
[70] https://t.me/tass_agency/268889
[71] https://www.facebook.com/GeneralStaff.ua/posts/pfbid02hvYvDy7aPcowjP6Tsya7omtQuMduZK4BxRneMhrCoeGS3QAt7hqV1imtW95tbpMhl; https://www.facebook.com/GeneralStaff.ua/posts/pfbid0ZQNnW7qYx5knBHTJJEMhKw5Pv7uasPG8q3kCjpbFVJCyBTcvZbRe1rmXEpKtL8Sql
[72] https://www.facebook.com/GeneralStaff.ua/posts/pfbid02hvYvDy7aPcowjP6Tsya7omtQuMduZK4BxRneMhrCoeGS3QAt7hqV1imtW95tbpMhl; https://www.facebook.com/GeneralStaff.ua/posts/pfbid02qUUyqRxjK1MKSTh5hjknbf7xGDXZtrB7U2H3AL28KceNo6rYyFVjy4RaN3ia8QU9l; https://www.facebook.com/GeneralStaff.ua/posts/pfbid0ZQNnW7qYx5knBHTJJEMhKw5Pv7uasPG8q3kCjpbFVJCyBTcvZbRe1rmXEpKtL8Sql
[73] https://t.me/voin_dv/10501
[74] https://www.facebook.com/GeneralStaff.ua/posts/pfbid02qUUyqRxjK1MKSTh5hjknbf7xGDXZtrB7U2H3AL28KceNo6rYyFVjy4RaN3ia8QU9l; https://www.facebook.com/GeneralStaff.ua/posts/pfbid0ZQNnW7qYx5knBHTJJEMhKw5Pv7uasPG8q3kCjpbFVJCyBTcvZbRe1rmXEpKtL8Sql ; https://www.facebook.com/OperationalCommandSouth/posts/pfbid0Qq9EbNXg5qVq7Fx1RzSwwBBHN4LKfCmCivGE9yu6kRAN7CaGxVvsXdwhjPWFnsqTl ; https://t.me/SJTF_Odes/11146
[75] https://t.me/police_kh_region/30583 ; https://t.me/mvs_ukraine/41155
[76] https://t.me/dnipropetrovskaODA/15592 ; https://t.me/dnipropetrovskaODA/15593 ; https://t.me/dnipropetrovskaODA/15594 ; https://t.me/dnipropetrovskaODA/15597
[77] https://t.me/rogozin_do/6348
[78] https://understandingwar.org/backgrounder/russian-offensive-campaign-assessment-may-22-2024 ; https://ria dot ru/20221111/rogozin-1830870951.html ; https://isw.pub/UkrWar061523 ; https://isw.pub/UkrWar070423 ; https://isw.pub/UkrWar080223 ; https://www.understandingwar.org/backgrounder/russian-offensive-campaign-assessment-november-7 ; https://www.understandingwar.org/backgrounder/russian-offensive-campaign-assessment-october-24 ; https://www.bbc.com/russian/news-67454788 ; https://www.understandingwar.org/backgrounder/russian-offensive-campaign-assessment-july-14-2024 ; https://understandingwar.org/backgrounder/russian-offensive-campaign-assessment-august-2-2024
[79] https://t.me/tass_agency/268936 ; https://t.me/dva_majors/50871 ; https://t.me/BalitskyEV/3803 ; https://t.me/dva_majors/50881
[80] https://t.me/voin_dv/10501
[81] https://t.me/bayraktar1070/2636 ; https://t.me/vysokygovorit/17101 ; https://t.me/milinfolive/129407
[82] https://t.me/tass_agency/268819; https://t.me/tass_agency/268890; https://t.me/tass_agency/268911 ; https://t.me/tass_agency/268921; https://t.me/tass_agency/268943
[83] https://t.me/otukharkiv/942
[84] https://t.me/otukharkiv/960; https://www.rbc dot ru/rbcfreenews/66cdbaea9a7947f9c51ad6ca
[85] https://tass dot ru/politika/21708225 ; https://t.me/tass_agency/269011
[86] https://www.understandingwar.org/backgrounder/russian-offensive-campaign-assessment-august-27-2024 ; https://armyinform.com dot ua/2024/08/27/navishho-bilorus-postijno-musuye-temu-kordonu-poyasnyly-v-dpsu/; https://isw.pub/UkrWar082624
[87] https://www.understandingwar.org/backgrounder/russian-offensive-campaign-assessment-august-27-2024
Flair Airlines says it's offering flights for a loonie. Experts say it needs to get real
Vp says deal is a way to give back to customers, expert calls strategy a 'loss leader'.
Flair Airlines offers $1 flights — some experts warn it’s a gimmick
Social sharing.
Would you buy a plane ticket for a loonie? Flair Airlines hopes so.
The ultra low-cost carrier says it has launched a $1 base airfare deal for passengers flying back to Canada from sun destinations — but some experts are skeptical of whether the airline can sustain the promo.
Flair says the $1 airfares, currently reserved for northbound flights from Mexico, the U.S., Jamaica and the Dominican Republic, are now offered permanently on its website.
The airline is offering $1 flights back to Canada from airports in Cancun and Puerto Vallarta, Orlando and Las Vegas. The deal applies to flights from down south to airports in Calgary, Edmonton, Montreal, Saint John, Vancouver, Toronto and Kitchener-Waterloo, Ont., and London, Ont.
Eric Tanner, vice-president of revenue management and network planning at Flair, said the deal is a way to "give back to our customers."
"We're coming up on the beginning of our winter season where we stop flying quite as much domestically and we start flying quite a bit more internationally."
- What do you think of this $1 base airfare deal? Let us know at [email protected]
He said those flights full when southbound, but that the aircraft are "quite empty" when they come back to Canada — so the deal incentivizes passengers to travel back up north. When the spring season begins, the deal will apply to southbound flights.
"These flights essentially have no opportunity cost. They are flying full south and empty north," he said. "And so even if we put four people onto that flight who otherwise wouldn't have taken it, that's great. We're happy about that."
Flair isn't the first carrier to try the $1 deal — Porter Airlines offered a similar promo for domestic flights in January 2023. And WestJet announced an UltraBasic Fare without carry-ons, seat selection and rewards points in June.
While the deal applies only to select flights, the airline said it plans to offer more routes throughout the year. The company stressed in a news release the deal is "not a one-time gimmick."
'It's a gimmick'
Others disagree.
"It's a gimmick," said Ian Lee, an associate professor of management at Carleton University's Sprott School of Business.
"This is essentially a loss leader. You see this in grocery stores and in retail. They're doing this to try to generate, drive business to the site and get people comfortable with flying on this airline.
"They won't be able to sustain it for very long because the operating cost per customer is significant," Lee added, saying the airline would need to be consistently putting customers in almost every seat for the move to be sustainable.
"That requires a relationship with the consumer. Air Canada has it, WestJet has it, Porter has it. These smaller discount airlines are trying to build consumer loyalty ... and it's a struggle in this industry."
Flair, one of the last ultra-low-cost airlines still operating in Canada, has significant debt. Court documents from earlier this year showed that it owed $67 million to the Canada Revenue Agency in import duties.
Four of its planes were repossessed in March 2023 after the company allegedly missed rent payments to a leasing manager, which amounted to millions of dollars.

Flair Airlines' flights cancelled after 4 planes seized in ‘commercial dispute’
The carrier announced on Wednesday it would be looking for investors to inject cash into the business to restructure its finances, helping to address its debt. It's also trying to grow its 20-plane fleet.
'The bill could be pretty expensive'
Some travellers at Toronto's Pearson International Airport were receptive to the Flair deal, while others said thanks, but no thanks.
"Make it to as many destinations as you can for a buck? I'm good with it. I'll certainly take advantage of that offer," said Max Kirsch.
"There's no such thing as a free lunch. But with that being said, a dollar for the flight, how much can they really boost your baggage fees?"
Others were less enthusiastic.

A $1 flight: The perfect deal? Or too good to be true?
"I'd be very suspicious about a $1 fare myself," said John Kangur. His wife Ella-Mai called the deal too good to be true.
"Many of these cheap flights, there's baggage fees, there's check-in fees, there's this fee, there's this fee. There's restrictions on time travel when, where. So we go with the airlines that we're comfortable with," she said.
Flair acknowledged the base fare is only one component of the ticket's total cost. Fees for baggage and seat selection will still apply, regardless of the promo, as will other airport fees that are collected each time you travel.
"You need to buy tickets, you have to buy baggage, carry-on baggage, checked baggage. You have to pay money for your seat, pay money for all types of others ancillary services," said John Gradek, an aviation industry expert at McGill University.

Airline fees on the rise as airfares drop
Additional and optional charges are detailed on Flair's website.
The carrier charges between $29 and $89 plus tax for carry-on bags, with the fee depending on whether you add the bag online, at the airport or at the gate.
A 10-kilogram checked bag costs between $29 and $59 plus tax. A 23-kilogram checked bag costs between $49 and $79 plus tax, and that cost goes up for bags checked at the airport (and with each subsequent 23-kilogram bag that is checked).
Standard seat selection on a Flair flight costs between $15 and $25 plus tax, with other charges for seats at the front of the plane, seats with extra legroom and seats in the emergency exit row.
- WestJet announces UltraBasic fare with no carry-ons, no seat choice and no points
Plus, most airports in Canada charge an "airport improvement fee" that adds to the final cost of your trip. For example, Toronto Pearson's fee is $35 for originating passengers and $7 for connecting passengers.
"The bill could be pretty expensive," said Gradek. "So be careful in terms of what you actually are buying all-in if you buy that $1 fare."
ABOUT THE AUTHOR
Jenna Benchetrit is the senior business writer for CBC News. She writes stories about Canadian economic and consumer issues, and has also recently covered U.S. politics. A Montrealer based in Toronto, Jenna holds a master's degree in journalism from Toronto Metropolitan University. You can reach her at [email protected].
With files from Nisha Patel and Laura MacNaughton
Related Stories

IMAGES
COMMENTS
TRIPS. Article 67 - Technical Cooperation. In order to facilitate the implementation of this Agreement, developed country Members shall provide, on request and on mutually agreed terms and conditions, technical and financial cooperation in favour of developing and least-developed country Members. Such cooperation shall include assistance in the ...
We would like to show you a description here but the site won't allow us.
7 TRIPS Article 67 Reports (2022) Reporting data •414 programmes of Technical Assistance reported delivered by Members in 2022 reporting period. •7 Members delivered substantive programmes to beneficiary countries. •5 International and Regional Organisations submitted reports containing 411 programmes of Technical Assistance. •813 of these programmes were reported in sufficient detail ...
Article 6 72 of TRIPS Agreement Article 67 creates the basis for an international regime between its members concerning international technical cooperation. There are no mandatory rules or methods of cooperation imposed on developed country members under this provision. However, the duty on developed countries to cooperate is
monitoring of compliance with the obligat ion of Article 67 of the Agreement on Trade- Related Aspects of Intellectual Property Rights (TRIPS Agreement), developed country Members have agreed to present descriptions of their relevant technical and financial cooperation programmes and to update
6This right, like all other rights conferred under this Agreement in respect of the use, sale, importation or other distribution of goods, is subject to the provisions of Article 6. filing date or, where priority is claimed, at the priority date of the application. 2.
As agreed by the TRIPS Council in the context of Article 67 of the TRIPS Agreement (IP/C/M/8, paragraphs 37 and 38). Specification by the notifying developed country Member of contact points for technical cooperation on TRIPS. Developed country WTO Members: One-time initial notification, and updates as necessary.
Article 67 of the TRIPS Agreement requires developed countries to provide technical assistance, "on request and on mutually agreed terms and conditions", to developing and least-developed countries to help address such gaps. Developing countries like Ghana should use this provision and approach developed countries and international ...
Article on technical cooperation.3 In the end the Article is now addressed to developed country Members and is for the benefi t of all developing and least-developed country Members. B. Implementation as a Goal Art. 67 facilitates the implementation of TRIPS in less-developed country Members.
AUSTRALIA. Addendum. The following communication, dated 14 October 2016, is being circulated at the request of the delegation of Australia. _____ Australia is committed to sharing information with other WTO Members on its technical and financial cooperation activities in the area of intellectual property rights in fulfilment of Article 67 of the TRIPS Agreement. This is a full report on ...
A further advantage inherent within TRIPS is the 'flexibility' offered to all members in interpreting various articles of the agreement (Vandoren, 2001). Article 27.3, for example, allows members to exclude certain inventions and subject matter from patentability, and permits the protection of others - such as plant varieties - through ...
Library of Congress Cataloging-in-Publication Data. A handbook on the WTO TRIPS Agreement / edited by Antony Taubman, Hannu Wager, and Jayashree Watal. p. cm. ISBN 978-1-107-02316-1 (Hardback) - ISBN 978-1-107-62529-7 (Paperback) 1. Intellectual property (International law) 2. Agreement on Trade-Related Aspects of Intellectual Property Rights ...
The TRIPS Agreement binds all Members of the WTO (see Article II.2 of the WTO Agreement)." The TRIPS Agreement was amended through the Protocol of 6 December 2005 that entered into force on 23 January 2017. The amendment inserted a new Article 31bis into the Agreement as well as an Annex and Appendix. These provide the legal basis for WTO ...
To help facilitate implementation of the TRIPS Agreement, Article 67 requires developed country members to provide, on request and on mutually agreed terms and conditions, technical and financial cooperation in favour of developing and least developed country (LDC) members. The deadline to submit applications is April 21, 2023.
The WTO Intellectual Property (IP) Division has opened the application process for a workshop on Article 67 of the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) to be held at the WTO on 12-13 June 2023. To help facilitate implementation of the TRIPS Agreement, Article 67 requires developed country members to provide, on […]
The Secretariat of the World Trade Organization (WTO) is organizing the Workshop on Article 67 of the TRIPS Agreement: Technical Cooperation, taking place in Geneva, Switzerland, on 12-13 June 20 The eligible candidates (civil servants of Montenegro) need to duly complete and submit the online application form, which is available at TRIPS ...
While Articles 7 and 8 have acquired influence at a policy level through the Doha Ministerial Declaration and the Declaration on TRIPS and Public Health, their use within the World Intellectual Property Organisation (WIPO) Development Agenda and the Anti-Counterfeiting Trade Agreement (ACTA) has resulted in both an enhanced status within the ...
Unlike the United Nations, there is no separate GATT or WTO treaty series. In less recent publications, you may also see a citation to a WTO publication called The Legal Texts: The Results of the Uruguay Round of Multilateral Trade Negotiations (Cambridge, U.K.; New York, N.Y.: Cambridge University Press, [2010] 1999) Reserve K4600.A35 R473 2010.
ARTICLE 67 TRIP REDUCTION 25-67-1: INTENT AND PURPOSE: Use of the private automobile in southern California has created serious traffic congestion and air quality problems. At the regional and local levels, government agencies have adopted policies and programs aimed at reducing the number of single passenger vehicles traveling along the region ...
Satellite images have been released in the aftermath of a Ukrainian drone attack on an ammunition depot in Russia's western Voronezh region. The photos, dated July 5 and July 7, are from ...
A Russian battalion commander from the 123rd Motorized Rifle Brigade (2nd Luhansk People's Republic Army Corps [LNR AC]) operating in the Siversk direction told Kremlin newswire TASS in an August 28 article that Russian forces in the area first conduct artillery and first-person view (FPV) drone strikes against Ukrainian positions and then ...
Ultra low-cost Flair Airlines says it has launched a $1 base airfare deal for passengers flying back to Canada from sun destinations — but some experts are skeptical of whether the airline can ...
Monuments & Statues (67) Architectural Buildings (36) Specialty Museums (31) Historic Walking Areas (4) Sports Complexes (5) Points of Interest & Landmarks (34) Theaters (11) ... It was a nice and relaxing trip into the nature after a crammed with excursions day in Voronezh and Voronezh area. Read more. Review of: Voronezh State Nature ...
Voronezh Oblast. Voronezh Oblast is in Russia's Chernozemye region, bordering Ukraine to the southwest, Belgorod Oblast to the west, Kursk Oblast to the northwest, Lipetsk Oblast to the north, Tambov Oblast to the northeast, Ulyanovsk Oblast to the northeast, Volgograd Oblast to the east, and Rostov Oblast to the south. Overview. Map. Directions.