
Personalize Your Experience
Log in or create an account for a personalized experience based on your selected interests.
Already have an account? Log In
Free standard shipping is valid on orders of $45 or more (after promotions and discounts are applied, regular shipping rates do not qualify as part of the $45 or more) shipped to US addresses only. Not valid on previous purchases or when combined with any other promotional offers.
Home / RSV / Treating RSV in adults: What to expect

Treating RSV in adults: What to expect
RSV is mild for most adults — but life-threatening for some. Here's how the virus is managed, from at-home treatments to hospitalization.
Please login to bookmark
Username or Email Address
Remember Me

For most adults, respiratory syncytial virus (RSV) isn’t high on their list of worries. This contagious virus is so common that almost everyone has had it at least once before the age of 2 — and several times in adulthood as well.
For most people, the cold-like symptoms of RSV are mild. They include a stuffy or runny nose, cough, and low fever. But for adults at high risk, including people 65 and older, and those with heart or lung issues, RSV can become a life-threatening condition.
Here’s what you can expect when it comes to RSV treatment.
Mild cases can be managed at home
RSV often feels like a common cold — and it’s treated the same too. Medical experts recommend that you take these self-care steps to manage mild symptoms:
- Drink plenty of fluids.
- Take acetaminophen (Tylenol, others) as needed.
- Use a saline solution to rinse mucus from your nose.
- Stay on top of regular medicines that help manage chronic heart or lung conditions.
When it’s time to see a healthcare professional
Like a common cold, RSV generally gets better in about 1 to 2 weeks. If your symptoms don’t get better, call your doctor or other healthcare professional.
Don’t delay if you continue to feel worse. These symptoms may need emergency medical attention right away:
- Shortness of breath.
- Blue or gray skin or lips.
- High fever.
- Chest pain.
What a doctor will do for RSV
Healthcare teams can test for RSV, COVID-19 and the flu with a blood test or swab in your nose. Tests also can diagnose RSV complications.
If you test positive for COVID-19 or the flu, your healthcare team can offer specific treatments for these viruses. But there isn’t a standard treatment for RSV. Your doctor will likely manage your symptoms the same way whether or not you test positive for RSV.
How RSV is managed in a clinic or hospital depends on how serious the symptoms are and whether you have other risk factors, like chronic heart disease or a weakened immune system.
Treatments may include:
- IV fluids. In a hospital, you will probably receive an IV to keep you hydrated.
- Medicines. There is medication available to treat RSV, which may be given by mouth or IV. You also may need antibiotics if the RSV has spread to a bacterial infection like pneumonia.
- Oxygen. You will likely be given oxygen to help you breathe more easily. Most people wear a nasal cannula, which is a thin plastic tube that delivers oxygen right to the nose.
- Mechanical ventilation. In rare cases, you may be put on a breathing machine.
Bottom line: Don’t wait to get medical help if your symptoms continue to get worse. If you’re 65 or older, have chronic heart or lung disease, or have a weakened immune system, you may be at high risk of serious RSV.
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.

Discover more RSV content from articles, podcasts, to videos.
You May Also Enjoy

Privacy Policy
We've made some updates to our Privacy Policy. Please take a moment to review.
Skip to content
How to Treat RSV at Home and When to Go to the Doctor
Published on Nov 16, 2023
Primary Care Locations

As we navigate another RSV season, common-sense prevention tips, plus new preventive medications and vaccines can make a big difference for little ones at risk for getting the sickest. Learn more about how to prevent RSV and, if your child does get it, how to ease their discomfort and get through the worst of it at home, if possible.
What is RSV?
RSV is a virus that causes a miserable cold with thick mucus and a cough that can last a month. You can get the infection at any age and more than once in your lifetime. RSV tends to be particularly tough on babies and toddlers who catch it because the first time around is usually the worst.
In most cases, RSV symptoms are mild enough to be managed at home. But there are some red flags to watch out for that might require a trip to the doctor.
How to prevent RSV
Practice trusted infection prevention steps! This means good handwashing and staying home when sick to prevent the spread of germs.
WHAT TO KNOW ABOUT NATIONAL SHORTAGE OF BEYFORTUS
How CHOP is responding to the national RSV medication shortage and keeping your baby safe.
Get any available preventive medications or vaccinations before the RSV season starts. In addition to vaccines available for pregnant people and adults over 60, a new preventive medication is now recommended by the Centers for Disease Control and Prevention (CDC) for babies before or during their first RSV season. The medication's name is Beyfortus (Nirsevimab). It is a single dose “monoclonal antibody” given in one shot into the muscle. It puts antibodies into the bloodstream that will help babies fight the virus. It decreases the chances of getting very sick and landing in the hospital from RSV.
How to tell if my child has RSV
Signs of RSV typically peak on days three, four and five of illness. They may include:
- Loss of appetite
How to treat RSV at home
Like all colds, there is no medication to cure RSV. However, here's how you can ease your child's discomfort at home:
- Clear up congestion. If your little one can’t (or won’t) blow their nose, put a drop or two of nasal saline in each nostril. Use a suction device like a bulb syringe to pull out the discharge. Warning: Excessive bulb suctioning can be irritating to the nose. Sometimes the saline alone is enough to promote sneezing, which will shoot out the mucus.
- Pick up steam. Run a cool mist humidifier in your child’s bedroom and give steamy baths. The water vapor loosens congestion. Note: Use a cool mist humidifier rather than a vaporizer, which is a burn hazard.
- Let honey help. If your child is at least a year old, try giving them honey to help relieve their cough. Honey works as well as popular over-the-counter cough medications without the side effects.
- Relieve pain. Give acetaminophen (if over 2 months of age) or ibuprofen (if over 6 months of age) as needed for fever or discomfort. These medications can help ease muscle aches, headaches and sore throats. For ear pain, you can also put a warm washcloth over the ear.
- Stay hydrated. For infants, breast milk or formula are best for hydration because of their nutrients. For older children, mix it up. Make sure there is salt and sugar in their fluids if they are not eating. Apple juice, water, popsicles, milk, soup, and foods like watermelon and cucumbers are also great sources of hydration. Milk will not make mucus worse.
How to know if a medical visit is needed
So how do you know when to take your child for medical care? Trust your instincts when trying to decide if a child’s cold requires medical attention.
Navigating Sick Season
Frequent illness is a normal part of childhood. Focus on what you can control and use these tips to help navigate the inevitable illnesses that circulate every year.
If you are not certain, call your child’s pediatrician’s office. You can also use the CHOP symptom checker to help you determine how serious their symptoms are. If your child has other medical conditions, has a weakened immune system, or sees a specialist, be sure to check with their specialty care team.
In general, keep an eye out for these signs of a more serious case:
- Rapid breathing
- Using extra muscles to breathe (watch for them using their shoulders or stomach to breathe, and look for the skin between the ribs getting sucked in with each breath)
- Grunting at the end of each breath
- Nostrils flaring with each breath
- Pale or blue lips/mouth
- Inability to breathe and drink at the same time
- Dehydration. Most kids urinate every three to six hours or so. You know your child’s baseline. If you struggle to keep them hydrated and they don't pee as much as usual, they need medical attention. Other signs of dehydration include a dry mouth, lethargy and lack of tears when crying.
- Pain: Like all colds, a child with RSV might develop ear infections, pneumonia or a sinus infections. Pay attention to complaints of ear pain, chest pain or sinus pain.
The bottom line
Don't panic! If your child is eligible, you can be proactive and take advantage of the antibody treatment to help prevent serious illness. If they do get sick, take steps to ease your child’s discomfort and get through the worst of it at home, use your judgement, and watch for the above warning signs.
We’ll get through this together!
Contributed by: Naline Lai, MD, FAAP , Julie Kardos, MD, FAAP , Lori Handy, MD, MSCE , Kathleen Filograna, MD, FAAP
Stay in Touch
Are you looking for advice to keep your child healthy and happy? Do you have questions about common childhood illnesses and injuries? Subscribe to our Health Tips newsletter to receive health and wellness tips from the pediatric experts at Children's Hospital of Philadelphia, straight to your inbox. Read some recent tips .

With our patient portal you can schedule appointments, access records, see test results, ask your care provider questions, and more.

Subscribe to Health Tips
Subscribe to our Health Tips enewsletter to receive health and wellness tips from the pediatric experts at CHOP.
You Might Also Like
Signs of respiratory distress in children.
Detailed information on the signs of respiratory distress in children.

Calming Your Child's Cough
Catching a cold is a normal part of being a child. Coughing that comes along with the cold is a key part of the body’s recovery process.
Respiratory syncytial virus (RSV)
On this page, risk factors, complications.
Respiratory syncytial virus (RSV) causes infections of the lungs and respiratory tract. It's so common that most children have been infected with the virus by age 2. Respiratory syncytial (sin-SISH-ul) virus can also infect adults.
In adults and older, healthy children, respiratory syncytial virus (RSV) symptoms are mild and typically mimic the common cold. Self-care measures are usually all that's needed to relieve any discomfort.
RSV can cause severe infection in some people, including babies 12 months and younger (infants), especially premature infants, older adults, people with heart and lung disease, or anyone with a weak immune system (immunocompromised).
Products & Services
- A Book: Mayo Clinic Family Health Book
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Signs and symptoms of respiratory syncytial virus infection most commonly appear about four to six days after exposure to the virus. In adults and older children, RSV usually causes mild cold-like signs and symptoms. These may include:
- Congested or runny nose
- Low-grade fever
- Sore throat
In severe cases
RSV infection can spread to the lower respiratory tract, causing pneumonia or bronchiolitis — inflammation of the small airway passages entering the lungs. Signs and symptoms may include:
- Severe cough
- Wheezing — a high-pitched noise that's usually heard on breathing out (exhaling)
- Rapid breathing or difficulty breathing — the person may prefer to sit up rather than lie down
- Bluish color of the skin due to lack of oxygen (cyanosis)
Infants are most severely affected by RSV . Signs and symptoms of severe RSV infection in infants include:
- Short, shallow and rapid breathing
- Struggling to breathe — chest muscles and skin pull inward with each breath
- Poor feeding
- Unusual tiredness (lethargy)
- Irritability
Most children and adults recover in one to two weeks, although some might have repeated wheezing. Severe or life-threatening infection requiring a hospital stay may occur in premature infants or in anyone who has chronic heart or lung problems.
RSV and COVID-19
Because RSV and coronavirus disease 2019 (COVID-19) are both types of respiratory viruses, some symptoms of RSV and coronavirus disease 2019 (COVID-19) can be similar. In children, COVID-19 often results in mild symptoms such as fever, runny nose and cough. For adults with COVID-19 , symptoms may be more severe and may include trouble breathing.
Having RSV may lower immunity and increase the risk of getting COVID-19 — for kids and adults. And these infections may occur together, which can worsen the severity of COVID-19 illness.
If you have symptoms of a respiratory illness, your doctor may recommend testing for COVID-19 .
When to see a doctor
Seek immediate medical attention if your child — or anyone at risk of severe RSV infection — has difficulty breathing, a high fever, or a blue color to the skin, particularly on the lips and in the nail beds.
From Mayo Clinic to your inbox
Respiratory syncytial virus enters the body through the eyes, nose or mouth. It spreads easily through the air on infected respiratory droplets. You or your child can become infected if someone with RSV coughs or sneezes near you. The virus also passes to others through direct contact, such as shaking hands.
The virus can live for hours on hard objects such as countertops, crib rails and toys. Touch your mouth, nose or eyes after touching a contaminated object and you're likely to pick up the virus.
An infected person is most contagious during the first week or so after infection. But in infants and those with weakened immunity, the virus may continue to spread even after symptoms go away, for up to four weeks.
By age 2, most children will have been infected with respiratory syncytial virus, but they can get infected by RSV more than once. Children who attend child care centers or who have siblings who attend school are at a higher risk of exposure and reinfection. RSV season — when outbreaks tend to occur — is the fall to the end of spring.
People at increased risk of severe or sometimes life-threatening RSV infections include:
- Infants, especially premature infants or babies who are 6 months or younger
- Children who have heart disease that's present from birth (congenital heart disease) or chronic lung disease
- Children or adults with weakened immune systems from diseases such as cancer or treatment such as chemotherapy
- Children who have neuromuscular disorders, such as muscular dystrophy
- Adults with heart disease or lung disease
- Older adults, especially those age 65 and older
Complications of respiratory syncytial virus include:
- Hospitalization. A severe RSV infection may require a hospital stay so that doctors can monitor and treat breathing problems and give intravenous (IV) fluids.
- Pneumonia. RSV is the most common cause of inflammation of the lungs (pneumonia) or the lungs' airways (bronchiolitis) in infants. These complications can occur when the virus spreads to the lower respiratory tract. Lung inflammation can be quite serious in infants, young children, older adults, immunocompromised individuals, or people with chronic heart or lung disease.
- Middle ear infection. If germs enter the space behind the eardrum, you can get a middle ear infection (otitis media). This happens most frequently in babies and young children.
- Asthma. There may be a link between severe RSV in children and the chance of developing asthma later in life.
- Repeated infections. Once you've had RSV , you could get infected again. It's even possible for it to happen during the same RSV season. However, symptoms usually aren't as severe — typically it's in the form of a common cold. But they can be serious in older adults or in people with chronic heart or lung disease.
Respiratory syncytial virus can infect anyone. But premature babies and young infants, as well as older adults, with heart or lung disease or a weakened immune system are at higher risk of severe infection.
Protection for babies and high-risk young children
Two main options exist to help prevent young infants from getting severe RSV . One is an antibody product given to the infant. The other is an RSV vaccine for pregnant people to help protect their baby from birth through 6 months of age. Both are approved by the U.S. Food and Drug Administration (FDA). You and your healthcare professional can discuss which option is best to protect your child.
Antibody product called nirsevimab (Beyfortus). This antibody product is a single-dose shot given in the month before or during RSV season. It's for babies younger than 8 months born during or entering their first RSV season. Nirsevimab also can be given to children 8 months through 19 months old who are at higher risk of severe RSV disease through their second RSV season. In the U.S., the RSV season typically is November through March, but it varies in Florida, Alaska, Hawaii, Puerto Rico, Guam and other U.S. Pacific island territories.
In rare situations, when nirsevimab is not available or a child is not eligible for it, another antibody product called palivizumab may be given. But palivizumab requires monthly shots given during the RSV season, while nirsevimab is only one shot. Palivizumab is not recommended for healthy children or adults.
- Vaccine for pregnant people. The FDA approved an RSV vaccine called Abrysvo for pregnant people to prevent RSV in infants from birth through 6 months of age. A single-dose shot of Abrysvo can be given sometime from 32 weeks through 36 weeks of pregnancy during September through January in the U.S.
Vaccine for older adults
Older adults have weaker immune systems, especially those with ongoing conditions, such as heart or lung disease. To help prevent RSV infection, the FDA approved RSV vaccines for adults age 60 and older.
The CDC recommends that adults age 60 and older talk with their healthcare professional about getting an RSV vaccine, especially if they're at higher risk of getting severe RSV . Two vaccines are available for this age group: Abrysvo and Arexvy. The CDC does not recommend one over the other. Each is a single-dose shot.
Talk with your healthcare team about the benefits and risks of RSV vaccines for your situation.
Lifestyle habits
These lifestyle habits can help prevent the spread of this infection:
- Wash your hands often. Teach your children the importance of hand-washing.
- Avoid exposure. Cover your mouth and nose when you cough or sneeze. Limit your baby's contact with people who have fevers or colds.
- Keep things clean. Make sure kitchen and bathroom countertops, doorknobs, and handles are clean. Put used tissues in the trash right away.
- Don't share drinking glasses with others. Use your own glass or disposable cups when you or someone else is sick. Label each person's cup.
- Don't smoke. Babies who are exposed to tobacco smoke have a higher risk of getting RSV and potentially more-severe symptoms. If you do smoke, never do so inside the house or car.
- Wash toys regularly. Do this especially when your child or a playmate is sick.
Oct 04, 2023
- Kliegman RM, et al. Respiratory syncytial virus. In: Nelson Textbook of Pediatrics. Elsevier; 2020. https://www.clinicalkey.com. Accessed Oct. 22, 2020.
- Ferri FF. Respiratory syncytial virus. In: Ferri's Clinical Advisor 2021. Elsevier; 2021. https://www.clinicalkey.com. Accessed Oct. 22, 2020.
- Hurley LP, et al. Primary care physicians' perspectives on respiratory syncytial virus (RSV) disease in adults and a potential RSV vaccine for adults. Vaccine. 2019; doi:10.1016/j.vaccine.2018.12.031.
- Chen X, et al. The microbial coinfection in COVID-19. Applied Microbiology and Biotechnology. 2020; doi:10.1007/s00253-020-10814-6.
- Respiratory syncytial virus infection (RSV): Symptoms and care. Centers for Disease Control and Prevention. https://www.cdc.gov/rsv/about/symptoms.html. Accessed Oct. 22, 2020.
- Respiratory syncytial virus infection (RSV): RSV prevention. Centers for Disease Control and Prevention. https://www.cdc.gov/rsv/about/prevention.html. Accessed Aug. 31, 2023.
- Respiratory syncytial virus infection (RSV): RSV transmission. Centers for Disease Control and Prevention. https://www.cdc.gov/rsv/about/transmission.html. Accessed Dec. 21, 2020.
- AskMayoExpert. Respiratory syncytial virus (RSV) infection (child). Mayo Clinic; 2019.
- AskMayoExpert. Respiratory syncytial virus (RSV) immunoglobulin series. Mayo Clinic; 2020.
- Nam HH, et al. Respiratory syncytial virus infection in adults. BMJ. 2019; doi:10.1136/bmj.l5021.
- American Academy of Pediatrics. Policy Statement ― Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014; doi:10.1542/peds.2014-1665. Reaffirmed 2019.
- Respiratory syncytial virus (RSV) and human metapneumovirus infections. Merck Manual Professional Version. https://www.merckmanuals.com/professional/pediatrics/miscellaneous-viral-infections-in-infants-and-children/respiratory-syncytial-virus-rsv-and-human-metapneumovirus-infections. Accessed Oct. 22, 2020.
- Synagis (prescribing information). Medimmune, LLC; 2017. https://synagishcp.com/. Accessed Oct. 22, 2020.
- Respiratory syncytial virus infection (RSV): RSV in older adults and adults with chronic medical conditions. Centers for Disease Control and Prevention. https://www.cdc.gov/rsv/high-risk/older-adults.html. Accessed Dec. 29, 2020.
- Baughn JM (expert opinion). Mayo Clinic. Dec. 14 and Dec. 30, 2020.
- Respiratory syncytial virus infection (RSV): RSV in infants and young children. Centers for Disease Control and Prevention. https://www.cdc.gov/rsv/high-risk/infants-young-children.html. Accessed Dec. 29, 2020.
- Jefferson JM, et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: Recommendations of the Advisory Committee on Immunization Practices — United States, 2023. MMWR Morbidity and Mortality Weekly Report, 2023. 72:793-801.
- FDA approves first vaccine for pregnant individuals to prevent RSV in infants. U.S. Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approves-first-vaccine-pregnant-individuals-prevent-rsv-infants. Accessed Oct. 3, 2023.
- RSV in infants and young children. Centers for Disease Control and Prevention. https://www.cdc.gov/rsv/high-risk/infants-young-children.html. Accessed Oct. 3, 2023.
- Respiratory syncytial virus (RSV) preventive antibody: Immunization information statement (IIS). Centers for Disease Control and Prevention. https://www.cdc.gov/vaccines/vpd/rsv/immunization-information-statement.html. Accessed Oct. 3, 2023.
- RSV immunization for children 19 months and younger. Centers for Disease Control and Prevention. https://www.cdc.gov/vaccines/vpd/rsv/public/child.html. Accessed Oct. 3, 2023.
- Should I get the RSV vaccine during pregnancy? American College of Obstetricians and Gynecologists. https://www.acog.org/womens-health/experts-and-stories/ask-acog/should-i-get-the-rsv-vaccine-during-pregnancy. Accessed Oct. 3, 2023.
- Frequently asked questions about RSV vaccine for adults. Centers for Disease Control and Prevention. https://www.cdc.gov/vaccines/vpd/rsv/hcp/older-adults-faqs.html. Accessed Oct. 3, 2023.
- Huskins WC (expert opinion). Mayo Clinic. Oct. 2, 2023.
- Jacobson RM (expert opinion). Mayo Clinic. Oct. 3, 2023.
- Diseases & Conditions
- Respiratory syncytial virus (RSV) symptoms & causes
News from Mayo Clinic
Associated Procedures
- Chest X-rays
CON-XXXXXXXX
Help transform healthcare
Your donation can make a difference in the future of healthcare. Give now to support Mayo Clinic's research.
Cookies on GOV.UK
We use some essential cookies to make this website work.
We’d like to set additional cookies to understand how you use GOV.UK, remember your settings and improve government services.
We also use cookies set by other sites to help us deliver content from their services.
You have accepted additional cookies. You can change your cookie settings at any time.
You have rejected additional cookies. You can change your cookie settings at any time.
- Health and social care
- Public health
- Health protection
- Immunisation
- Respiratory syncytial virus (RSV): vaccination for older adults
- UK Health Security Agency
Your guide to the RSV vaccine for older adults
Updated 5 August 2024
Applies to England

© Crown copyright 2024
This publication is licensed under the terms of the Open Government Licence v3.0 except where otherwise stated. To view this licence, visit nationalarchives.gov.uk/doc/open-government-licence/version/3 or write to the Information Policy Team, The National Archives, Kew, London TW9 4DU, or email: [email protected] .
Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned.
This publication is available at https://www.gov.uk/government/publications/respiratory-syncytial-virus-rsv-vaccination-for-older-adults/your-guide-to-the-rsv-vaccine-for-older-adults
From 1 September 2024, those who turn 75 and those age 75 to 79 will be eligible for a free vaccine to protect them from respiratory syncytial virus ( RSV ).
RSV is an infectious disease of the airways and lungs. RSV infection often causes symptoms similar to a cold, including:
- sore throat
- a runny or blocked nose
It can also make you become wheezy or short of breath and lead to pneumonia and other life-threatening conditions. There is no specific treatment, and most infections will get better by themselves. Every year thousands of older adults need hospital care for RSV , and some of them will die. RSV can be more severe in people with medical conditions such as heart or lung disease or a weakened immune system.
RSV infection is common in young children but is most serious for small babies and for older people.
How RSV is spread
RSV infections can occur all year round but cases peak every winter.
RSV can spread through coughs and sneezes. You can help to prevent the spread of the virus by covering your mouth and nose when you cough or sneeze (ideally with a tissue, or else into the bend of your elbow), and you can wash your hands frequently to reduce the risk of picking up the virus. Even with these measures it can be difficult to avoid RSV infection.
The best way to protect yourself is to have the vaccine.
How the RSV vaccine works
Almost all older adults will have had several RSV infections during their life. A single dose of vaccine will help to boost protection as you reach an age group at highest risk of serious RSV infection. Unlike the flu vaccine you do not need to have the RSV vaccine every year.
Eligibility for the RSV vaccination
Everyone turning 75 years old on or after the 1 September 2024 will be offered a single dose of RSV vaccine. This is because older adults are more at risk of serious complications from RSV . You can still get the vaccine up to the day before you turn 80.
For the first year of the programme, the vaccine will also be offered to those who are already aged 75 to 79 years on 1 September 2024 as part of a catch up programme.
If you are not yet 75 the NHS will invite you for vaccination once you turn 75.
Having the RSV vaccine at the same time as other vaccines
Shingles and pneumococcal vaccines.
You may be offered your shingles or pneumococcal vaccine at the same time as the RSV vaccine, it is safe to do so and reduces the number of appointments you will need to get protected from these diseases.
Flu and COVID-19 vaccines
RSV isn’t normally arranged to be given at the same appointment as your COVID-19 or flu vaccines.
In certain clinical circumstances, your doctor or nurse can safely offer them at the same time.
Those who should not have the RSV vaccine
Almost everybody can have the vaccine, but tell the doctor or nurse if you have ever had a serious allergy to a vaccine, or any of the ingredients.
You can read the patient information leaflet for the RSV vaccine called Abrysvo .
If you have a minor illness such as a cold you can receive the vaccine, but if you are very unwell or have a fever, the doctor or nurse may advise you to wait until you are better.
The protection offered by the RSV vaccine
The vaccine has been shown to reduce the chance of you suffering from RSV disease. As with all medicines, no vaccine is completely effective and some people may still get RSV despite having a vaccination. If you do get RSV , it should be less severe.
How long the RSV vaccine will protect you for
In the clinical studies, RSV vaccine provided good protection for at least 2 years and is expected to last longer.
Side effects from the RSV vaccine
You may get some soreness, redness or swelling at the site of the injection for a day or two after the vaccination.
In the first season of use in the US over 3 million doses were given. A nervous system condition that leads to weakness was reported, around 5 cases for every million doses given. The same condition also occurs after a number of common infections and some vaccines but overall it is safer for you to have the vaccine than to risk having a potentially serious RSV infection.
You can report suspected side effects to the Medicines and Healthcare products Regulatory Agency ( MHRA ):
- on the Yellow Card website
- by calling the free phone line: 0800 731 6789 (9am to 5pm, Monday to Friday)
- by downloading the Yellow Card App on iOS or Android
Translations and accessible versions of this information
This leaflet is available in the following languages: Albanian , Arabic , Bengali , Bulgarian , Chinese (simplified) , Chinese (traditional) , Dari , Estonian , Farsi , French , Greek , Gujarati , Hindi , Italian , Latvian , Lithuanian , Nepali , Panjabi , Pashto , Polish , Portuguese , Romanian , Romany , Russian , Somali , Spanish , Tagalog , Tigrinya , Turkish , Twi , Ukrainian , Urdu , Yiddish and Yoruba .
This leaflet is also available in a range of accessible formats: Audio , Braille , British Sign Language and Large Print .
RSV for Older Adults - British Sign Language
Further information
If you have further questions, speak to your practice nurse, GP or health team.
Read the patient information leaflet for the RSV vaccine Abrysvo .
Is this page useful?
- Yes this page is useful
- No this page is not useful
Help us improve GOV.UK
Don’t include personal or financial information like your National Insurance number or credit card details.
To help us improve GOV.UK, we’d like to know more about your visit today. Please fill in this survey (opens in a new tab) .
- Skip to main content
- Keyboard shortcuts for audio player
Your Health
- Treatments & Tests
- Health Inc.
- Public Health
A single-shot treatment to protect infants from RSV may be coming soon
Tarryn Mento

Each year, RSV infections send up to 80,000 kids under 5 to the hospital for emergency treatment. A new antibody treatment could protect the youngest kids — newborns and up infants up to 2 years old. Christoph Soeder/dpa/picture alliance via Getty I hide caption
Each year, RSV infections send up to 80,000 kids under 5 to the hospital for emergency treatment. A new antibody treatment could protect the youngest kids — newborns and up infants up to 2 years old.
Cheryl Meany, a high school teacher from Camillus, N.Y., was excited when she learned she was carrying twins in 2014. But her joy quickly turned to worry as doctors flagged several health concerns, including possible brain lesions.
So she needed a moment to process when her husband, a respiratory therapist, proposed enrolling the soon-to-be-born babies in an experimental study for an unrelated illness. It was a trial for a protective treatment for RSV or respiratory syncytial virus, a common respiratory virus that can be quite severe in young children.
"It took me aback, like 'What are you even talking about? I don't even know what you're asking me right now,'" Meany said.
That was in 2014, several years before the recent RSV surge overwhelmed hospitals across the country. But Meany was worried about the illness back then after seeing some of her friends' kids end up in the hospital from it. Up to 80,000 children under 5 are admitted for RSV each year.
So she enrolled her daughters in the trial for a monoclonal antibody that works to prevent RSV-induced lower respiratory tract infection in infants. Her decision helped move forward one of the most promising treatments to protect babies from severe impacts of RSV in decades.
In January, drugmakers AstraZeneca and Sanofi announced the U.S. Food and Drug Administration is officially reviewing their application to get the treatment – called nirsevimab – approved in the U.S., including results from the trial the Meany twins joined.
AstraZeneca said its third phase trial results showed its single-dose treatment was nearly 75% effective at preventing severe infection in babies throughout an RSV season. The data was published in March 2022 in the New England Journal of Medicine.

Before they were born in 2015, twins, Stella and Cassidy Meany (left to right), were enrolled in a trial for a preventative treatment for RSV. The treatment may soon be available to protect newborns against the respiratory illness. Cheryl Meany hide caption
Before they were born in 2015, twins, Stella and Cassidy Meany (left to right), were enrolled in a trial for a preventative treatment for RSV. The treatment may soon be available to protect newborns against the respiratory illness.
Dr. William Schaffner, medical director at the National Foundation for Infectious Diseases who was not involved in this research, said the results suggest nirsevimab could significantly reduce the numbers of babies that are hospitalized each year for RSV.
"The potential impact in assuring a healthy infancy for a very large proportion of the infants born here in the United States — and even beyond — is potentially very, very large," Schaffner said.
A form of 'passive immunization'
The drug – a long-lasting antibody injection – is intended for newborns or other infants facing their first RSV season, and for babies up to 24 months of age in their second RSV season, according to AstraZeneca's press release.
Dr. Joseph Domachowske, a pediatric infectious disease specialist at Upstate Medical University Hospital in Syracuse, helped launch the earliest phase of the nirsevimab study.
"RSV is the number one reason why infants and young children are hospitalized, not just in the U.S., but across the world," he said.
He explained that the antibody treatment is not a vaccine but is what scientists refer to as "passive immunization." The antibodies against RSV circulate in the infants' bodies protecting against the virus, should the child be exposed.
"It doesn't cause an immune response itself," he said, and it also doesn't cause the body to develop immune memory. "But it provides protection for a period of time until it wears off," he said. A similar type of treatment was used to help protect immunocompromised patients against COVID.
Domachowske, who also led the hospital's COVID-19 vaccine trial for kids , expects a greenlight from regulators in time to have nirsevimab available by the next RSV season in the fall. It has already been approved in Europe .
Long journey to an effective treatment
When Meany's daughters got their injections in January 2015, they were the first babies in the world to receive it, according to AstraZeneca.
Domachowske, a Meany family friend, said giving the twin babies protection against RSV was a significant moment after researchers had struggled for years to find a treatment to prevent RSV. Back in the 1960s, a different treatment, a vaccine candidate , was under study. But it made kids sicker from RSV – and two babies died from it.
"It really charged up the wrong half of the immune system," Domachowske said.
Progress didn't come until two decades later. In 1998, the FDA OK'd a monoclonal antibody for premature and high-risk babies. But Domachowske said changing medical guidelines since then have severely limited eligibility for this treatment, and, he said, its efficacy wasn't great.
"It has to be given monthly," Domachowske said. "And it's effective at preventing hospitalization, not effective at preventing infection."
That's where the research had been stuck for years until 2014, when Domachowske attended a medical conference in Argentina. A featured speaker dropped a massive discovery that a lot of RSV research focused on the wrong protein.
"Everyone is sitting there staring with their mouths gaping open like, 'This is why all of our work hasn't led to anything for decades," Domachowske said. "It was that impressive. And you can see the pharma people that were attending, taking notes, calling their colleagues saying, 'Stop, stop the work.'"
Not too long later, he injected Meany's daughters with an improved, longer-lasting monoclonal antibody that protects babies through an RSV season with one shot.

The Meany twins were the first in the world to get shots of nirsevimab during early trials when they were infants. They had no side effects and no symptoms of RSV. Cheryl Meany hide caption
The Meany twins were the first in the world to get shots of nirsevimab during early trials when they were infants. They had no side effects and no symptoms of RSV.
The twin girls, Cassidy and Stella, are now 8 years old and like to compete in ninja warrior contests — they race through obstacle courses that feature ladders, monkey bars and overturned Bosu balls.
Meany said the girls never had complications from the shot and never displayed symptoms of RSV. She is proud of the role they played in medical history.
"This matters, and this matters for kids everywhere, not just kids here,'" Meany said.
Domachowske said the girls may have gotten RSV in later seasons after the effects of the treatment had worn off. But since older children's immune systems are stronger, symptoms weren't noticeable.
A welcome RSV prevention tool
Physicians and infectious disease specialists welcome the potential approval of the treatment.
Schaffner of the National Foundation for Infectious Diseases said if it were already approved in the U.S., nirsevimab would've helped curb the high rate of infections seen this season, one of the worst recent seasons for the disease.
"This recent surge would have been remarkably blunted," he said.
Dr. Vandana Madhavan , clinical director of pediatric infectious disease at Mass General for Children said the monoclonal antibody is a significant achievement in the fight against RSV.
"This is a huge step forward," she said.
- monoclonal antibody
- childhood vaccination
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 25 September 2023
The road to approved vaccines for respiratory syncytial virus
- Tracy J. Ruckwardt ORCID: orcid.org/0000-0002-0007-7169 1
npj Vaccines volume 8 , Article number: 138 ( 2023 ) Cite this article
9315 Accesses
15 Citations
4 Altmetric
Metrics details
After decades of work, several interventions to prevent severe respiratory syncytial virus (RSV) disease in high-risk infant and older adult populations have finally been approved. There were many setbacks along the road to victory. In this review, I will discuss the impact of RSV on human health and how structure-based vaccine design set the stage for numerous RSV countermeasures to advance through late phase clinical evaluation. While there are still many RSV countermeasures in preclinical and early-stage clinical trials, this review will focus on products yielding long-awaited efficacy results. Finally, I will discuss some challenges and next steps needed to declare a global victory against RSV.
Similar content being viewed by others
Learning from the past: development of safe and effective COVID-19 vaccines
A systematic review of SARS-CoV-2 vaccine candidates
A computational approach to design a polyvalent vaccine against human respiratory syncytial virus
Introduction.
Since the discovery of respiratory syncytial virus (RSV, originally called Chimpanzee Coryza Agent) in 1956, much has been learned about its pathogenesis and the impact of RSV disease in humans. RSV presents a substantial burden in young infants across diverse settings 1 , 2 . It is the most common cause of acute lower respiratory tract infection (LRTI) and hospitalization in children under 2 years of age 3 . In 2019, it was estimated to cause 33 million cases of acute LRTI, 3.6 million hospitalizations, and over 100,000 deaths in children under 5 years of age 4 . More than 97% of RSV-attributable deaths in children under 5 occur in low- and middle-income countries (LMIC), a high proportion of them occurring in the community rather than in a hospital 4 , 5 . Most hospitalizations occur in infants less than 6 months old and an estimated 6.6 million acute LRTI infections, 1.4 million hospital admissions, and more than 45,000 RSV-attributable overall deaths occur in this age group globally 4 , 6 . While most children hospitalized with RSV have no known risk factors, prematurity, chronic lung disease, congenital heart disease and several other factors predispose to severe disease 7 , 8 . RSV infection in early life has been linked to childhood asthma and impaired lung function, and in a prospective study, avoiding infection in the first year of life substantially lowered the risk of childhood asthma 9 , 10 .
At the opposite end of the age spectrum, older adults are also at risk for severe RSV disease, particularly the frail elderly or those with comorbid conditions 11 , 12 , 13 , 14 , 15 . The annual attack rate for older adults generally ranges between 3% to 10%, resulting in an estimate of over 177,000 hospitalization and 14,000 deaths in older adults in the United States every year 14 , 16 . The burden of RSV in older adults is underestimated because sampling procedures typically used for RSV diagnosis (RT-PCR from nasopharyngeal swabs) have limitations in adults who can have lower viral titers than infected children. Using a wider variety of samples including saliva, serum, and sputum dramatically increases the cases of RSV diagnosed among adults hospitalized with acute respiratory infection 17 , 18 , 19 , 20 . Severe RSV disease in older adults has long-term effects, which often include the worsening of prior conditions. Hospital readmission rates are high within 30 days after discharge, and there is substantial health care utilization through 6 months. Patients often require home health services or long-term care facility placement, and there is an increased risk for mortality within the first year 21 .
In summary, RSV exerts a substantial burden on the health of young infants and older adults globally, with impacts that extend well beyond acute infection. Vaccines and other countermeasures that can be used broadly to combat the impact of RSV on these high-risk populations are urgently needed.
Respiratory syncytial virus
RSV is a member of the Pneumoviridae family 22 . It has a negative-sense genome encoding 11 proteins. The nucleoprotein (N), phosphoprotein (P), polymerase (L) and M2–1 transcription processivity factor comprise the ribonucleocapsid, which is encased in an endoskeleton of envelope-associated matrix (M) protein. The M protein lattice coordinates a densely packed viral envelope, studded with the fusion (F), attachment (G), and small hydrophobic (SH) membrane proteins 23 , 24 , 25 . Other RSV proteins include the nonstructural proteins NS1 and NS2, and a regulatory factor translated from a second, overlapping open reading frame in the M2 gene called M2–2. RSV buds from infected cells as filamentous particles but breaks down to asymmetric and spherical particles over time 26 . The G and F glycoproteins are the primary targets of neutralizing antibodies. Despite containing a small central conserved domain, G has the highest genetic diversity, enabling the segregation of viral sequences into two subtypes, A and B, each of which contains multiple genotypes 27 . While one subtype may dominate during a season, A and B subtypes generally cocirculate. F is a type I viral fusion protein, synthesized as single-chain polypeptides that are cleaved by host proteases and displayed as trimers on the viral envelope. RSV F is unusual in that it contains two polybasic cleavage sites, resulting in the release of a 27 amino acid fragment before formation of the mature protein. F is an absolute requirement for viral fusion with the host cell and has a high level of genetic and antigenic conservation 28 . Most protective antibodies target the F protein, and the F-specific antibody palivizumab (Synagis®) has been used to protect high-risk infants from disease since 1998 29 , 30 .
Despite limited antigenic variability in the most protective antigen, RSV is a seasonal and ubiquitous cause of human disease. RSV infection does not generate durable immunity against reinfection, similar to what is seen for other respiratory viruses 31 , 32 . Responses to infection are limited, and reinfection is common 33 , 34 . As seen for other respiratory viruses, changes in human behavior and mitigation efforts after the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) interrupted RSV transmission and disease 35 . RSV attack rates were low in 2020, followed by an atypical outbreak in May of 2021 in the Northern hemisphere or November of 2021 in South Africa 36 , 37 . By 2022, RSV disease surged a few months earlier than its typical seasonal pattern and joined influenza and SARS-CoV-2 in driving high rates of respiratory disease and hospitalization 38 . The continued impact of RSV on morbidity and mortality makes the development and implementation of effective countermeasures critical, despite disrupted epidemiology and seasonal disease patterns during the pandemic 39 .
Early RSV vaccines
The RSV vaccine field encountered tragedy shortly after discovery of the virus. Based on technology of the time, a formalin-inactivated (FI) RSV candidate was the first to be tested. Immunity elicited by FI-RSV primed for more severe disease after natural RSV infection – 80% of infected FI-RSV immunized children were hospitalized and two died as a result 40 . Vaccination with FI parainfluenza virus vaccine candidates did not similarly prime for enhanced respiratory disease (ERD) after RSV infection, suggesting a role of RSV-specific immunity. The unexpected failure of FI-RSV made developers balk and approach testing, particularly in antigen-naïve infants, with a high degree of caution. The ERD outcome has been linked to the induction of antibodies with weak neutralizing activity responsible for complement fixation and immune complex deposition and Th2-biased CD4 + T cell responses, a profile that should be carefully avoided when vaccinating antigen-naïve young infants against RSV 41 . There is no precedent for enhanced disease in infants that have had a prior RSV infection, thus live-attenuated vaccines present one of the lowest risks for eliciting unfavorable immune responses in RSV-naïve infants. A major challenge for this approach has been balancing attenuation and immunogenicity 41 .
For several decades, few live attenuated and purified fusion protein or subunit-based vaccine candidates advanced to late-phase testing only to yield unsatisfying efficacy results 41 . While much was learned about RSV biology, epidemiology, and the challenges of protecting the most vulnerable populations, the response to RSV disease has relied primarily on supportive care. Prophylactic monthly administration of palivizumab during the RSV season has proven useful since 1998 to prevent severe disease in a small population of premature and at-risk infants, but it has not demonstrated a therapeutic benefit 42 , 43 .
Structure-based vaccine design
Structural determination of the two major conformations of the F protein offered some explanations for the failure of prior vaccines to protect. The structure of postfusion F (postF) was determined in 2011. PostF is highly stable and displays known sites of recognition for neutralizing antibodies including palivizumab and its more potent derivative motavizumab 44 , 45 . Determining the structure of prefusion F (preF) proved more difficult, and a monoclonal antibody (mAb) was needed to lock the protein in this metastable active conformation 46 . While the F protein undergoes a dramatic and irreversible conformational transformation to postF during fusion, a substantial portion of the protein remains relatively unchanged such that four described antigenic sites (I, II, III, IV) are displayed on both forms of the protein. In addition to these “shared” epitopes, the metastable preF displays conformation-specific antigenic sites referred to as sites Ø and V on the apex 41 , 46 . Monoclonal antibody discovery efforts to map the antigenic surface of preF have shown that sites Ø and V are targets for the most potently neutralizing antibodies, thus representing major sites of vulnerability 47 , 48 , 49 . Therefore, to elicit the most potent antibodies and in turn, confer the most protection, F-based vaccines need to retain antigenic sites Ø and V. This was the basis for the first prefusion-stabilized subunit vaccine candidate called DS-Cav1 50 . DS-Cav1 was evaluated in a phase 1 clinical trial, providing the first proof-of concept in humans for potent elicitation of neutralizing activity and high potency antibodies targeting the antigenic sites unique to preF 51 , 52 . Other stabilization solutions followed DS-Cav1, demonstrating superior elicitation of neutralizing activity with a variety of stabilizing mutations 53 , 54 , 55 .
DS-Cav1 activates memory B cells specific for all known antigenic sites, while postF vaccination activates B cells recognizing shared sites I-IV. As a result of eliciting lower potency antibodies, postF vaccination results in a higher fold-increase in binding than neutralization after immunization, and overall lower potency neutralizing activity compared to DS-Cav1 49 , 56 , 57 . Thus, the limited immunogenicity and efficacy of many prior vaccines can be linked to the presentation of postF lacking the most critical targets of vulnerability. This is most evident for the “lot 100” FI-RSV vaccine – the preparation method used to produce the vaccine resulted in the absence of preF on the surface, altering the antigenicity away from that of the infectious virus 58 . Several F-based subunit vaccines evaluated were known or revealed to be postF, and elicited immunity could not recapitulate the nature of post-infection human sera, where antibodies specific for preF are responsible for most neutralizing activity 59 , 60 , 61 , 62 , 63 . An F-based nanomeric micelle vaccine candidate made in Sf9 insect cells was found to display a variety of F conformations and retain an intermediate level of binding to preF only binding antibodies 64 , 65 . It did not achieve sufficient efficacy for protection from LRTI in late-phase testing in older adults, or for protection of infants of vaccinated mothers in the PREPARE phase 3 trial (NCT02624947). The vaccine elicited an 18.6-fold increase in F-binding IgG, but only a 2- to 3-fold increase in neutralizing activity, a profile like that seen following vaccination with postF antigens 66 .
Determination of the preF structure, and the demonstration that vaccines retaining the preF structure preserved neutralization-sensitive epitopes and elicited supranormal levels of neutralizing activity was a game changer. The field shifted to mAbs targeting preF, and vaccines designed to display the neutralization-sensitive sites Ø and V on the preF apex. The changing landscape for vaccines and mAbs curated by PATH ( https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/ ), and a recent comprehensive review detailing all candidates being tested in humans provide broader perspectives that are not covered by this review 67 .
Late-phase and approved vaccines
The RSV field is at an unprecedented moment. Late-phase trials of vaccine candidates based on stabilization of the preF protein or leveraging our improved understanding of RSV biology to elicit protective immune responses have delivered or will soon have late-phase efficacy results. Two mAbs and a maternal subunit vaccine are the most advanced candidates for protection in infants, while several vaccine candidates are vying for the older adult market. These most advanced candidates (Fig. 1 ) are further discussed below.
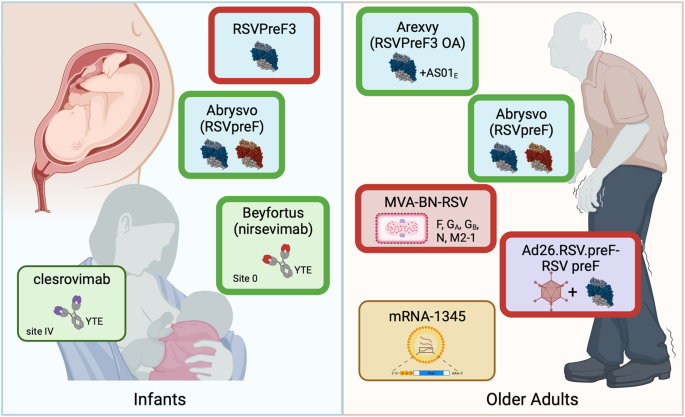
For protection of infants from RSV disease, the nirsevimab and clesrovimab half-life extended (YTE) mAbs are market-approved or nearing completion of phase 3 evaluation, respectively. The maternal RSVpreF bivalent subunit vaccine Abrysvo has been FDA approved for administration between 32 and 36 weeks of pregnancy, while development of the A subtype RSVPreF3 was stopped due to a safety signal. For protection of older adults, both the AS01 E -adjuvanted subtype A subunit Arexvy and unadjuvanted bivalent subunit Abrysvo have been approved by the FDA, while development of Ad26.RSV.preF-RSV preF and MVA-BN-RSV vaccines for older adults has been stopped. mRNA-1345 is nearing phase 3 completion. A thick green border indicates market approved, and thick red border indicates that product development and testing has been discontinued. Figure created using biorender.com.
Infant protection from RSV disease
Protection of young infants from severe disease prior to their first exposure can be achieved by bolstering neutralizing antibody responses. One way to achieve this is by direct administration of potent neutralizing antibodies. Currently, the use of the site II-targeting mAb palivizumab is restricted to neonates with extreme prematurity ( < 29 weeks’ gestation) or infants with other selected risk factors due to high cost and the need for monthly administration. It is expected to be replaced by more potent antibodies with extended half-life “YTE” mutations (M252Y/S254T/T256E) in the Fc portion so a single administration can confer protection for an entire RSV season 68 . Increased potency and durability of protection make extending coverage to all infants an achievable goal.
The most advanced mAb, nirsevimab (trade name Beyfortus), binds antigenic site Ø and has >50-fold higher neutralizing activity than palivizumab 69 . Nirsevimab has a comparable safety and side effect profile to palivizumab when compared in palivizumab-eligible infants, and in a phase 2b primary cohort of healthy late-preterm and term infants, a single 50 or 100 mg dose (based on size) prior to the RSV season demonstrated 75% protection against medically attended RSV-associated LRTI 70 , 71 . It was similarly protective when evaluated in healthy preterm infants 72 . A phase 3 trial (NCT03979313) showed an efficacy of 76% against medically attended LRTI, 77% against hospitalization due to RSV-associated LRTI, and 79% against very severe medically attended LRTI 73 . A pooled analysis demonstrated that prophylaxis with nirsevimab was 80% effective against medically attended LRTI through to 150 days post-enrollment. Based on pharmacokinetic data, the efficacy is expected to be similar in full-term infants as well as those born prematurely or with chronic lung or congenital heart disease 74 . Importantly, despite providing durable protection, nirsevimab does not appear to prevent the development of effective immune responses in infants later infected with RSV 75 . While rare resistance mutations were identified in RSV F protein sequences isolated from infected infants that received nirsevimab, more than 99% of the F protein sequences obtained remained susceptible 76 . Nirsevimab has been market-approved in Europe and the UK and was approved by the Food and Drug Administration (FDA) in July 2023. The Advisory Committee on Immunization Practices (ACIP) has recommended that a dose be given to all infants younger than 8 months entering their first RSV season and a second season dose for high risk groups, and has voted to include nirsevimab in the Vaccines for Children program which provides vaccines at no cost to those who might not be vaccinated because of inability to pay 77 .
Site IV-targeting clesrovimab (MK-1654) is a second half-life extended mAb undergoing phase 3 testing. It recognizes a quaternary epitope, preferentially binding preF over postF and demonstrating high in vivo potency against subtype A and B RSV 78 . Data are available from testing in healthy adults, and modeling studies predict high levels of protection from LRTI through 150 days at a dose of ≥ 75 mg in infants 79 , 80 , 81 . A phase 2b/3 efficacy study in healthy pre- and full-term infants (NCT04767373) is estimated to be completed in 2024, and a phase 3 comparison to palivizumab in high-risk infants (NCT04938830) in 2026.
The alternative approach to protect infants through the first several months of life is maternal vaccination, which relies on the transplacental transfer of neutralizing antibodies. This approach is used successfully for tetanus, influenza, and pertussis, and more recently COVID-19 vaccines 82 , 83 . Despite not meeting its primary success criteria, the PREPARE trial was the first to demonstrate some short-term efficacy in the offspring of vaccinated women and the feasibility of this approach for RSV F-based vaccines 66 , 84 . Two preF-stabilized subunit vaccines were next into Phase 3 testing, RSVpreF and RSVPreF3. RSVpreF is bivalent, comprising equal amounts of A and B subtype preF. After testing in healthy adults, it was tested in a phase 2b trial where doses of 120 or 240 µg with or without aluminum hydroxide adjuvant were given to healthy women between 24 and 36 weeks’ gestation 85 , 86 . Fold-rise in maternal neutralizing antibody in an interim analysis ranged between 11–15-fold for RSV A and 14–18-fold for RSV B. The ratio of RSV-specific antibody transfer through the placenta from mother to infant (ratio of cord blood neutralizing titer to mother’s neutralizing titer) ranged from 1.4 to 2.1 across viral subtypes and vaccine regimens. Observed efficacy point estimates for RSVpreF were 85% against medically attended RSV-associated LRTI and 92% against severe RSV-associated LRTI 86 . In a prespecified interim analysis of a phase 3 trial (NCT04424316), maternal immunization with 120 µg of unadjuvanted RSVpreF (trade name Abrysvo) resulted in vaccine efficacy of 82% against medically attended severe RSV-associated LRTI within 90 days after birth and 69% within 180 days after birth. The estimate for protection from medically attended LRTI was 57% and 51% within 90 and 180 days, respectively 87 . After maternal immunization for prevention of RSV disease in infants under the age of 6 months was recommended by the Vaccines and Related Biological Products Advisory Committee (VRBPAC) in May 2023, the use of Abrysvo between 32 and 36 weeks of pregnancy was FDA approved in August.
The RSV A subtype preF subunit candidate RSVPreF3 was tested at multiple doses in nonpregnant women before further testing at 60 and 120 µg doses during pregnancy 88 , 89 . Neutralizing activity against RSV A and B rose 13–15-fold and 11–13-fold, respectively, and at-birth antibody transfer ratios were between 1.6 and 1.9. However, due to safety signals in the pivotal phase 3 trial (NCT04605159), enrollment and vaccination in clinical trials evaluating RSVPreF3 were stopped in February 2022 89 .
Given the advancement of both next generation mAbs and maternal vaccines for the protection of young infants, these approaches may complement each other depending on vaccination practices, which may vary greatly by location. The cost of mAbs may limit use in healthy infants in some countries, and it may be difficult to achieve high uptake of maternal antibodies in others. Importantly, both passive antibodies and maternal vaccination have demonstrated a high level of protection in young infants and steer clear of the profile of immunity that was associated with ERD following FI-RSV immunization. These interventions offer hope that protection can be extended beyond only the highest risk infants to protect all infants from both the acute and long-term sequelae of severe RSV disease.
Older adult protection from RSV disease
Several vaccines for protection of older adults are completing phase 3 pivotal trials. RSVPreF3 OA, comprising the RSVPreF3 subunit tested for maternal immunization and AS01 E adjuvant, was the first RSV vaccine approved by the FDA for prevention of lower respiratory tract disease in adults 60 years of age or older. FDA approval comes after evaluation of RSVpreF3 in young adults (18–40-year-olds) and older adults (60–80-year-olds) at 30, 60, or 120 µg doses with no adjuvant, or with AS01 B or AS01 E adjuvant. Mean fold-increases in geometric mean titers (GMTs) above baseline for RSV A ranged from 5.5 to 9.6 on day 31 and were durable, ranging from 2.7 to 4.4-fold above baseline at 14 months 90 . A dose effect was observed, and 120 µg of RSVPreF3 with AS01 E was selected for further development as RSVPreF3 OA, trade name Arexvy. In a planned interim efficacy assessment of the phase 3 trial (NCT04886596), Arexvy had an overall efficacy of 83% against RSV-related lower respiratory tract disease (LRTD). Efficacy was 94% against severe RSV-related LRTD, and 72% against RSV-related acute respiratory infection. Neutralizing activity increased 10-fold for RSV A and 9-fold for RSV B in the immunogenicity cohort, and high efficacy was observed against LRTD due to both subtypes A and B RSV and across coexisting conditions and frailty status 91 . Arexvy was approved by the FDA for use in adults over 60 on May 3, 2023, making it the world’s first approved RSV vaccine.
Abrysvo, recently approved for prevention of RSV disease in infants of vaccinated mothers, also protects older adults from disease. The bivalent RSVpreF subunit vaccine candidate was tested at multiple doses with and without alum adjuvant in healthy adults between 18 and 49 years of age, eliciting an 11–17-fold (RSV A) and 10 to 20-fold (RSV B) geometric mean fold rise in neutralizing activity across doses and formulations with no benefit for the addition of alum. Titers were maintained 4 to 5-fold above baseline at 12 months post-vaccination 85 . RSVpreF was tested with and without alum at the same doses (60 µg, 120 µg, and 240 µg) alone or with seasonal inactivated influenza vaccine (SIIV). RSV neutralizing titers rose by 7 to 15-fold at one month and remained 3 to 5-fold elevated at 12 months post-vaccination. While RSVpreF was well-tolerated and highly immunogenic with or without SIIV, immune responses to SIIV trended lower when RSVpreF was co-administered 92 . RSVpreF was also tested at doses between 60 and 240 µg in healthy adults between 65 and 85 years old with either alum or CpG/alum. At all doses and formulations, geometric mean titers (GMTs) rose between 5–14-fold against RSV A and B, with a 2–4-fold elevation above baseline at 12 months. CpG/alum did not enhance the response to RSVpreF, and again, responses to SIIV were similar or trended slightly lower when the vaccines were co-administered 93 . Efficacy of Abrysvo (120 µg of unadjuvanted RSVpreF) was 87% against symptomatic RSV in an experimental human challenge study in adults 18 to 50 years of age, concomitant with lower viral shedding in vaccinated than nonvaccinated participants 94 . Finally, in an interim analysis of the phase 3 pivotal trial evaluating Abrysvo in adults over 60 years of age (NCT05035212), efficacy was 67% and 86% against RSV-associated LRTI with at least 2 and at least 3 signs and symptoms, respectively. Efficacy against RSV-associated acute respiratory illness was 62% 95 . Abrysvo was approved by the FDA for prevention of RSV disease in adults over the age of 60 at the end of May 2023. Following FDA approval of both Arexvy and Abrysvo, the ACIP has recommended a single dose of either subunit vaccine for adults over the age of 60 using shared clinical decision-making 96 .
A vaccine candidate using replication-defective adenovirus 26 to encode stabilized preF (Ad26.RSV.preF) resulted in a reduction in RSV infections, viral load, and disease severity when tested in a human challenge model in adults aged 18–50 years old 97 . It was later combined with recombinant preF protein into a single vaccine, Ad26.RSV.preF-RSV preF, which was tested in adults over the age of 65 in a phase 2b trial (NCT03982199). It elicited a 12-fold rise in neutralizing activity against RSV A and 9-fold rise against RSV B fold and had efficacy between 70% and 80% based on clinical case definitions ranging from mild to severe 98 . Ad26.RSV.preF-RSV preF has been under evaluation in a phase 3 trial since 2021 (NCT04908683), but an announcement of discontinuation of the trial and older adult program for the vaccine was made in March 2023.
Two additional vaccines are completing phase 3 pivotal trials for protection in older adults. The COVID-19 pandemic demonstrated that mRNA-lipid nanoparticle (mRNA-LNP) is a viable option to quickly deliver vaccines for infectious diseases at scale 99 , 100 . Leveraging innovations in mRNA-LNP technology and the superiority of preF immunogens, mRNA-1345 encodes membrane-anchored preF. No peer-reviewed immunogenicity or efficacy data for mRNA-1345 are available, but a press release from the sponsor indicates that efficacy in the ongoing phase 3 pivotal trial in adults aged 60 and older (NCT05127434) is 84% against LRTD with 2 or more symptoms 101 . Breakthrough therapy designation has been granted to mRNA-1345, and the BLA submission to the US FDA is expected to be completed in 2023. The MVA-BN-RSV vaccine candidate uses a nonreplicating modified vaccinia Ankara (MVA) virus to deliver multiple RSV antigens (F, N, M2–1, and G from both A and B subtypes) 102 . Serum neutralizing activity increased less than 2-fold by two weeks after MVA-BN-RSV vaccination, but most vaccinees had an increase in T cell responses to multiple of the five encoded RSV antigens 103 . In a human challenge study, MVA-BN-RSV increased neutralizing activity 2-fold for RSV A and 1.6-fold for RSV B, and point estimates for efficacy ranged between 10% and 89% depending on infection definition 104 . In July of 2023, the phase 3 trial testing MVA-BN-RSV in adults 60 years of age or older (NCT05238025) failed to hit a co-primary endpoint with efficacy estimates against severe LRTD with at least 3 symptoms at 42.9%, and development of the vaccine has been stopped.
Interestingly, most candidates achieving phase 3 efficacy results in older adults elicit at least a 10-fold increase in neutralizing activity and similar durability through one year. As a result, evidence across multiple late-phase trials suggests that this level of immunogenicity confers substantial protection ( ~ 80% or more against severe disease outcomes). Beyond completion of the phase 3 pivotal trials, more work is needed to understand the durability of protection, the need for booster immunizations, and how the vaccines will perform in the highest risk groups, including frail elderly and immunocompromised not represented in clinical trials. Post-marketing phase 4 studies are also needed to resolve concerns about possible safety signals seen in phase 3 trials and ensure a high level of safety as these vaccines are anticipated to be administered to millions of people annually. Further studies are also needed to determine whether responses to vaccines for other indications are affected by coadministration with RSV vaccines such that their clinical effectiveness is reduced. Finally, sharing of any available late-phase data despite the termination of vaccine programs in older adults and infants will be vital for our understanding of protective immunity and to inform future trial design and implementation of vaccines going forward (Fig. 1 ).
Remaining challenges
With several effective countermeasures against RSV now approved, we have not yet reached the end of the road. Critical challenges remain around protecting the remaining affected population of children over the age of 6 months and improving equity, education, and surveillance. Meeting these challenges will require contributions from people with many different types of expertise, including policy makers, science educators, and physicians serving high-risk populations. Additionally, as RSV vaccines may soon be distributed widely, we should take the opportunity to assess and ask questions that we may only be able to answer during this transitional time.
While the highest risk populations now have protective interventions, nearly half of RSV-associated hospitalizations and deaths occur in children between 6 months and five years of age 4 . Children over 6 months old are more capable of responding to active vaccination, and improvements in live-attenuated and gene-based vaccines may lead to the elicitation of responses to RSV that safely protect naïve young children 41 . As young and school-aged children are often responsible for transmission to infants and older adults, a vaccine that limits transmission could benefit other target populations. There should be a continued investment in protecting this at-risk, major transmitting population 105 , 106 . Evaluation of different types of vaccines, particularly if they contain RSV proteins in addition to F, may help reveal the contribution of antibody and T cell responses to other viral antigens as well as other immune mechanisms of protection from disease. This could include antibody effector functions beyond neutralization, and responses that exclusively occur at mucosal sites 31 , 107 . Based on the FI-RSV experience, it will be critical to avoid eliciting immune responses that could lead to immunopathology following infection, particularly in antigen-naïve infants 108 .
The overwhelming majority of deaths from RSV occur in LMIC, making it imperative that steps are taken to ensure access to interventions in places with the highest burden of disease. Many barriers to deployment in LMIC exist 109 . Low awareness of RSV and limited country-level data are major obstacles to defining the impact that interventions could have. Limited availability and costs of diagnostic testing contribute to the lack of information, which is important for understanding the full burden of disease and benefits of immunization 110 , 111 . Cost of goods will be another obstacle to preventive approaches in LMIC. Collaborations like the one established between the Bill & Melinda Gates foundation and Pfizer will enable faster and more equitable distribution of a maternal vaccine, and other public-private partnerships or the use of biosimilars may help drive down costs of vaccines less than $5 a dose, the target price for LMIC 109 . Education at the community level is critical in LMIC and globally to raise awareness of the impact of RSV. Increased awareness among health care professionals and communication of the benefits of prevention to high-risk groups prior to the implementation of vaccines will help engender trust and counter the rise in vaccine hesitancy. Efforts to increase patient involvement and communication infrastructure like the RSV Patient Advisory Board should be applauded and expanded 112 .
Widespread surveillance and sequencing efforts are also needed as vaccines and mAbs are implemented. Global viral evolution data, particularly from LMIC, and increased whole-genome data are critical knowledge gaps 27 , 113 . This is especially important during monotherapy deployment. While it was not intervention driven, natural changes in circulating viruses leading to ineffectiveness of the mAb suptavumab against B subtype RSV is a cautionary tale 114 . So far, sequencing efforts have revealed that nirsevimab escape variants are rare with no increase over time, but nirsevimab escape is possible and there is some natural variability in antigenic site Ø 28 , 115 , 116 , 117 . While several surveillance and sequencing programs exist, broadening our sequence databases and including whole genome sequences will offer insights into RSV evolution and biology as well as identify any impact of countermeasures. A unified nomenclature will aid such efforts 118 , 119 .
Finally, the SARS-CoV-2 pandemic shifted RSV epidemiology, bringing challenges but also offering some interesting opportunities 39 . As pneumococcal carriage rates remained relatively stable while viral seasonality was disrupted, one such opportunity was taken to demonstrate that RSV and human metapneumovirus (hMPV) are major contributors to community-acquired alveolar pneumonia, accounting for an estimated 49% and 13% of cases, respectively 120 . Interventions with high efficacy will also alter our decades-long relationship with RSV and have potential to improve overall lung health. This offers opportunities to ask questions about secondary effects of vaccination on long-term disease sequelae and pose questions that may become more difficult or impossible to answer once interventions become standard of care.
Concluding remarks
Despite tragedy, setbacks, and decades of work toward an RSV vaccine, stabilized preF transformed the field, leading to a crop of promising interventions to significantly reduce RSV-associated morbidity and mortality in high-risk populations. Next-generation monoclonal antibodies offer the possibility of protecting infants through a full season with a single immunization, and several preF-based vaccines boost neutralizing antibody responses by 10-fold or more and confer a high level of protection from severe disease outcomes in both young infants and the elderly. There is much excitement as these long-awaited interventions are approved and being deployed. Further effort should be directed to continued progress on other challenges, and to take advantage of one-time opportunities presented by the upcoming change in our long-standing relationship with RSV.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Langley, J. M. et al. Incidence of respiratory syncytial virus lower respiratory tract infections during the first 2 years of life: a prospective study across diverse global settings. J. Infect. Dis. 226 , 374–385 (2022).
PubMed PubMed Central Google Scholar
Blount, R. E. Jr., Morris, J. A. & Savage, R. E. Recovery of cytopathogenic agent from chimpanzees with coryza. Proc. Soc. Exp. Biol. Med. 92 , 544–549 (1956).
PubMed Google Scholar
Pneumonia Etiology Research for Child Health Study Group. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet 394 , 757–779 (2019).
Google Scholar
Li, Y. et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 399 , 2047–2064 (2022).
Mazur, N. I. et al. Global respiratory syncytial virus-related infant community deaths. Clin. Infect. Dis. 73 , S229–S237 (2021).
Parikh, R. C. et al. Chronologic age at hospitalization for respiratory syncytial virus among preterm and term infants in the United States. Infect. Dis. Ther. 6 , 477–486 (2017).
Hall, C. B. et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics 132 , e341–348, (2013).
Langley, G. F. & Anderson, L. J. Epidemiology and prevention of respiratory syncytial virus infections among infants and young children. Pediatr. Infect. Dis. J. 30 , 510–517 (2011).
Esteban, I., Stein, R. T. & Polack, F. P. A durable relationship: respiratory syncytial virus bronchiolitis and asthma past their golden anniversary. Vaccines (Basel) 8 , 201 (2020).
CAS PubMed Google Scholar
Rosas-Salazar, C. et al. Respiratory syncytial virus infection during infancy and asthma during childhood in the USA (INSPIRE): a population-based, prospective birth cohort study. Lancet 401 , 1669–1680 (2023).
Malloy, A. M., Falsey, A. R. & Ruckwardt, T. J. Consequences of immature and senescent immune responses for infection with respiratory syncytial virus. Curr. Top. Microbiol Immunol. 372 , 211–231 (2013).
Ackerson, B. et al. Severe morbidity and mortality associated with respiratory syncytial virus versus influenza infection in hospitalized older adults. Clin. Infect. Dis. 69 , 197–203 (2019).
Branche, A. R. et al. Incidence of respiratory syncytial virus infection among hospitalized adults, 2017-2020. Clin. Infect. Dis. 74 , 1004–1011 (2022).
Falsey, A. R., Hennessey, P. A., Formica, M. A., Cox, C. & Walsh, E. E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med . 352 , 1749–1759 (2005).
Falsey, A. R. et al. Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness. J. Infect. Dis. 209 , 1873–1881 (2014).
Sundaram, M. E. et al. Medically attended respiratory syncytial virus infections in adults aged >/= 50 years: clinical characteristics and outcomes. Clin. Infect. Dis. 58 , 342–349 (2014).
Li, Y. et al. Adjusting for case under-ascertainment in estimating rsv hospitalisation burden of older adults in high-income countries: a systematic review and modelling study. Infect. Dis. Ther. 12 , 1137–1149 (2023).
Onwuchekwa, C. et al. Under-ascertainment of Respiratory Syncytial Virus infection in adults due to diagnostic testing limitations: A systematic literature review and meta-analysis. J. Infect. Dis. 228 , 173–184 (2023).
Ramirez, J. et al. Diagnosis of respiratory syncytial virus in adults substantially increases when adding sputum, saliva, and serology testing to nasopharyngeal swab RT-PCR. Infect. Dis. Ther. 12 , 1593–1603 (2023).
McLaughlin, J. M. et al. Rates of medically attended RSV among US adults: a systematic review and meta-analysis. Open Forum Infect. Dis. 9 , ofac300 (2022).
Tseng, H. F. et al. Severe morbidity and short- and mid- to long-term mortality in older adults hospitalized with respiratory syncytial virus infection. J. Infect. Dis. 222 , 1298–1310 (2020).
Rima, B. et al. ICTV virus taxonomy profile: pneumoviridae. J. Gen. Virol. 98 , 2912–2913 (2017).
CAS PubMed PubMed Central Google Scholar
Conley, M. J. et al. Helical ordering of envelope-associated proteins and glycoproteins in respiratory syncytial virus. EMBO J. 41 , e109728 (2022).
Kiss, G. et al. Structural analysis of respiratory syncytial virus reveals the position of M2-1 between the matrix protein and the ribonucleoprotein complex. J. Virol. 88 , 7602–7617 (2014).
Liljeroos, L., Krzyzaniak, M. A., Helenius, A. & Butcher, S. J. Architecture of respiratory syncytial virus revealed by electron cryotomography. Proc. Natl Acad. Sci. USA 110 , 11133–11138 (2013).
Ke, Z. et al. The morphology and assembly of respiratory syncytial virus revealed by cryo-electron tomography. Viruses 10 , 446 (2018).
Rios Guzman, E. & Hultquist, J. F. Clinical and biological consequences of respiratory syncytial virus genetic diversity. Ther. Adv. Infect. Dis. 9 , 20499361221128091 (2022).
Hause, A. M. et al. Sequence variability of the respiratory syncytial virus (RSV) fusion gene among contemporary and historical genotypes of RSV/A and RSV/B. PLoS One 12 , e0175792 (2017).
The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 102 , 531–537 (1998).
Garegnani, L. et al. Palivizumab for preventing severe respiratory syncytial virus (RSV) infection in children. Cochrane Database Syst. Rev. 11 , CD013757 (2021).
Morens, D. M., Taubenberger, J. K. & Fauci, A. S. Rethinking next-generation vaccines for coronaviruses, influenzaviruses, and other respiratory viruses. Cell Host Microbe 31 , 146–157 (2023).
Yewdell, J. W. Individuals cannot rely on COVID-19 herd immunity: Durable immunity to viral disease is limited to viruses with obligate viremic spread. PLoS Pathog. 17 , e1009509 (2021).
Hall, C. B., Walsh, E. E., Long, C. E. & Schnabel, K. C. Immunity to and frequency of reinfection with respiratory syncytial virus. J. Infect. Dis. 163 , 693–698 (1991).
Ohuma, E. O. et al. The natural history of respiratory syncytial virus in a birth cohort: the influence of age and previous infection on reinfection and disease. Am. J. Epidemiol. 176 , 794–802 (2012).
Chow, E. J., Uyeki, T. M. & Chu, H. Y. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat. Rev. Microbiol 21 , 195–210 (2023).
Bardsley, M. et al. Epidemiology of respiratory syncytial virus in children younger than 5 years in England during the COVID-19 pandemic, measured by laboratory, clinical, and syndromic surveillance: a retrospective observational study. Lancet Infect. Dis. 23 , 56–66 (2023).
Izu, A. et al. All-cause and pathogen-specific lower respiratory tract infection hospital admissions in children younger than 5 years during the COVID-19 pandemic (2020-22) compared with the pre-pandemic period (2015-19) in South Africa: an observational study. Lancet Infect. Dis. 23 , 1031–1041 (2023).
Adams, G. et al. Viral lineages in the 2022 RSV surge in the United States. N. Engl. J. Med. 388 , 1335–1337 (2023).
Stein, R. T. & Zar, H. J. RSV through the COVID-19 pandemic: Burden, shifting epidemiology, and implications for the future. Pediatr. Pulmonol. 58 , 1631–1639 (2023).
Kim, H. W. et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89 , 422–434 (1969).
Ruckwardt, T. J., Morabito, K. M. & Graham, B. S. Immunological lessons from respiratory syncytial virus vaccine development. Immunity 51 , 429–442 (2019).
Caserta, M. T., O’Leary, S. T., Munoz, F. M., Ralston, S. L. & Committee On Infectious, D. Palivizumab Prophylaxis in Infants and Young Children at Increased Risk of Hospitalization for Respiratory Syncytial Virus Infection. Pediatrics 152 , e2023061803 (2023).
Sanders, S. L., Agwan, S., Hassan, M., van Driel, M. L. & Del Mar, C. B. Immunoglobulin treatment for hospitalised infants and young children with respiratory syncytial virus infection. Cochrane Database Syst. Rev. 8 , CD009417 (2019).
McLellan, J. S., Yang, Y., Graham, B. S. & Kwong, P. D. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J. Virol. 85 , 7788–7796 (2011).
Swanson, K. A. et al. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc. Natl Acad. Sci. USA 108 , 9619–9624 (2011).
McLellan, J. S. et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 340 , 1113–1117 (2013).
Gilman, M. S. et al. Rapid profiling of RSV antibody repertoires from the memory B cells of naturally infected adult donors. Sci. Immunol. 1 , eaaj1879 (2016).
Goodwin, E. et al. Infants infected with respiratory syncytial virus generate potent neutralizing antibodies that lack somatic hypermutation. Immunity 48 , 339–349.e335 (2018).
Mukhamedova, M. et al. Vaccination with prefusion-stabilized respiratory syncytial virus fusion protein induces genetically and antigenically diverse antibody responses. Immunity 54 , 769–780.e766 (2021).
McLellan, J. S. et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 342 , 592–598 (2013).
Crank, M. C. et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science 365 , 505–509 (2019).
Ruckwardt, T. J. et al. Safety, tolerability, and immunogenicity of the respiratory syncytial virus prefusion F subunit vaccine DS-Cav1: a phase 1, randomised, open-label, dose-escalation clinical trial. Lancet Respir. Med. 9 , 1111–1120 (2021).
Che, Y. et al. Rational design of a highly immunogenic prefusion-stabilized F glycoprotein antigen for a respiratory syncytial virus vaccine. Sci. Transl. Med. 15 , eade6422 (2023).
Joyce, M. G. et al. Iterative structure-based improvement of a fusion-glycoprotein vaccine against RSV. Nat. Struct. Mol. Biol. 23 , 811–820 (2016).
Krarup, A. et al. A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat. Commun. 6 , 8143 (2015).
Chang, L. A. et al. A prefusion-stabilized RSV F subunit vaccine elicits B cell responses with greater breadth and potency than a postfusion F vaccine. Sci. Transl. Med. 14 , eade0424 (2022).
Phung, E. et al. Elicitation of pneumovirus-specific B cell responses by a prefusion-stabilized respiratory syncytial virus F subunit vaccine. Sci. Transl. Med. 14 , eabo5032 (2022).
Killikelly, A. M., Kanekiyo, M. & Graham, B. S. Pre-fusion F is absent on the surface of formalin-inactivated respiratory syncytial virus. Sci. Rep. 6 , 34108 (2016).
Falloon, J. et al. An adjuvanted, postfusion F protein-based vaccine did not prevent respiratory syncytial virus illness in older adults. J. Infect. Dis. 216 , 1362–1370 (2017).
Langley, J. M. et al. A randomized, controlled, observer-blinded phase 1 study of the safety and immunogenicity of a respiratory syncytial virus vaccine with or without alum adjuvant. J. Infect. Dis. 215 , 24–33 (2017).
Graham, B. S. The journey to RSV vaccines - heralding an era of structure-based design. N. Engl. J. Med. 388 , 579–581 (2023).
Magro, M. et al. Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc. Natl Acad. Sci. USA 109 , 3089–3094 (2012).
Ngwuta, J. O. et al. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci. Transl. Med. 7 , 309ra162 (2015).
Krueger, S. et al. Structural characterization and modeling of a respiratory syncytial virus fusion glycoprotein nanoparticle vaccine in solution. Mol. Pharm. 18 , 359–376 (2021).
Patel, N. et al. Respiratory syncytial virus prefusogenic fusion (F) protein nanoparticle vaccine: Structure, antigenic profile, immunogenicity, and protection. Vaccine 37 , 6112–6124 (2019).
Madhi, S. A. et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N. Engl. J. Med. 383 , 426–439 (2020).
Mazur, N. I. et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect. Dis. 23 , e2–e21 (2023).
Dall’Acqua, W. F., Kiener, P. A. & Wu, H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J. Biol. Chem. 281 , 23514–23524 (2006).
Zhu, Q. et al. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci. Transl. Med. 9 , eaaj1928 (2017).
Domachowske, J. et al. Safety of nirsevimab for RSV in infants with heart or lung disease or prematurity. N. Engl. J. Med. 386 , 892–894 (2022).
Hammitt, L. L. et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N. Engl. J. Med . 386 , 837–846 (2022).
Griffin, M. P. et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N. Engl. J. Med. 383 , 415–425 (2020).
Muller, W. J. et al. Nirsevimab for prevention of RSV in term and late-preterm infants. N. Engl. J. Med . 388 , 1533–1534 (2023).
Simoes, E. A. F. et al. Efficacy of nirsevimab against respiratory syncytial virus lower respiratory tract infections in preterm and term infants, and pharmacokinetic extrapolation to infants with congenital heart disease and chronic lung disease: a pooled analysis of randomised controlled trials. Lancet Child Adolesc. Health 7 , 180–189 (2023).
Wilkins, D. et al. Durability of neutralizing RSV antibodies following nirsevimab administration and elicitation of the natural immune response to RSV infection in infants. Nat. Med. 29 , 1172–1179 https://doi.org/10.1038/s41591-023-02316-5 (2023).
Article CAS PubMed PubMed Central Google Scholar
Ahani, B. et al. Molecular and phenotypic characteristics of RSV infections in infants during two nirsevimab randomized clinical trials. Nat. Commun. 14 , 4347 (2023).
Jones, J. M. et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: recommendations of the advisory committee on immunization practices - United States, 2023. MMWR Morb. Mortal. Wkly Rep. 72 , 920–925 (2023).
Tang, A. et al. A potent broadly neutralizing human RSV antibody targets conserved site IV of the fusion glycoprotein. Nat. Commun. 10 , 4153 (2019).
Aliprantis, A. O. et al. A Phase 1 randomized, double-blind, placebo-controlled trial to assess the safety, tolerability, and pharmacokinetics of a respiratory syncytial virus neutralizing monoclonal antibody MK-1654 in healthy adults. Clin. Pharm. Drug Dev. 10 , 556–566 (2021).
CAS Google Scholar
Orito, Y. et al. A phase I study to evaluate safety, pharmacokinetics, and pharmacodynamics of respiratory syncytial virus neutralizing monoclonal antibody MK-1654 in healthy Japanese adults. Clin. Transl. Sci. 15 , 1753–1763 (2022).
Maas, B. M. et al. Forward and reverse translational approaches to predict efficacy of neutralizing respiratory syncytial virus (RSV) antibody prophylaxis. EBioMedicine 73 , 103651 (2021).
Etti, M. et al. Maternal vaccination: a review of current evidence and recommendations. Am. J. Obstet. Gynecol. 226 , 459–474 (2022).
Langel, S. N., Blasi, M. & Permar, S. R. Maternal immune protection against infectious diseases. Cell Host Microbe 30 , 660–674 (2022).
Blunck, B. N., Rezende, W. & Piedra, P. A. Profile of respiratory syncytial virus prefusogenic fusion protein nanoparticle vaccine. Expert Rev. Vaccines 20 , 351–364 (2021).
Walsh, E. E. et al. A randomized phase 1/2 study of a respiratory syncytial virus prefusion F vaccine. J. Infect. Dis. 225 , 1357–1366 (2022).
Simoes, E. A. F. et al. Prefusion F protein-based respiratory syncytial virus immunization in pregnancy. N. Engl. J. Med. 386 , 1615–1626 (2022).
Kampmann, B. et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N. Engl. J. Med . 388 , 1451–1464 (2023).
Schwarz, T. F. et al. Three dose levels of a maternal respiratory syncytial virus vaccine candidate are well tolerated and immunogenic in a randomized trial in nonpregnant women. J. Infect. Dis. 225 , 2067–2076 (2022).
Bebia, Z. et al. Safety and immunogenicity of an investigational respiratory syncytial virus vaccine (RSVPreF3) in mothers and their infants: a phase 2 randomized trial. J. Infect. Dis. 228 , 299–310 (2023).
Leroux-Roels, I. et al. Safety and immunogenicity of a respiratory syncytial virus prefusion F (RSVPreF3) candidate vaccine in older adults: phase 1/2 randomized clinical trial. J. Infect. Dis. 227 , 761–772 (2023).
Papi, A. et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N. Engl. J. Med . 388 , 595–608 (2023).
Falsey, A. R. et al. Phase 1/2 randomized study of the immunogenicity, safety, and tolerability of a respiratory syncytial virus prefusion F vaccine in adults with concomitant inactivated influenza vaccine. J. Infect. Dis. 225 , 2056–2066 (2022).
Baber, J. et al. A phase 1/2 study of a respiratory syncytial virus prefusion F vaccine with and without adjuvant in healthy older adults. J. Infect. Dis. 226 , 2054–2063 (2022).
Schmoele-Thoma, B. et al. Vaccine efficacy in adults in a respiratory syncytial virus challenge study. N. Engl. J. Med . 386 , 2377–2386 (2022).
Walsh, E. E. et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N. Engl. J. Med. 388 , 1465–1477 (2023).
Melgar, M. et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the advisory committee on immunization practices - United States, 2023. MMWR Morb. Mortal. Wkly Rep. 72 , 793–801 (2023).
Sadoff, J. et al. Prevention of respiratory syncytial virus infection in healthy adults by a single immunization of Ad26.RSV.preF in a human challenge study. J. Infect. Dis. 226 , 396–406 (2022).
Falsey, A. R. et al. Efficacy and safety of an Ad26.RSV.preF-RSV preF protein vaccine in older adults. N. Engl. J. Med. 388 , 609–620 (2023).
Chaudhary, N., Weissman, D. & Whitehead, K. A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 20 , 817–838 (2021).
Whitaker, J. A., Sahly, H. M. E. & Healy, C. M. mRNA vaccines against respiratory viruses. Curr. Opin. Infect. Dis. 36 , 385–393 (2023).
Carvalho, T. mRNA vaccine effective against RSV respiratory disease. Nat. Med . 29 , 755–756 (2023).
Samy, N. et al. Safety and immunogenicity of novel modified vaccinia Ankara-vectored RSV vaccine: A randomized phase I clinical trial. Vaccine 38 , 2608–2619 (2020).
Jordan, E. et al. Broad antibody and cellular immune response from a phase 2 clinical trial with a novel multivalent poxvirus-based respiratory syncytial virus vaccine. J. Infect. Dis. 223 , 1062–1072 (2021).
Jordan, E. et al. Reduced respiratory syncytial virus load, symptoms, and infections: a human challenge trial of MVA-BN-RSV vaccine. J. Infect. Dis. 20 , jiad108 (2023).
Korsten, K. et al. Contact With Young Children Increases the Risk of Respiratory Infection in Older Adults in Europe-the RESCEU Study. J. Infect. Dis. 226 , S79–S86 (2022).
Yamin, D. et al. Vaccination strategies against respiratory syncytial virus. Proc. Natl Acad. Sci. USA 113 , 13239–13244 (2016).
Bartsch, Y. C. et al. Antibody effector functions are associated with protection from respiratory syncytial virus. Cell 185 , 4873–4886.e4810 (2022).
Graham, B. S. Immunological goals for respiratory syncytial virus vaccine development. Curr. Opin. Immunol. 59 , 57–64 (2019).
Carbonell-Estrany, X., Simoes, E. A., Bont, L. J., Paes, B. A. & Collaborators, R. S. V. P. Prioritising respiratory syncytial virus prevention in low-income and middle-income countries. Lancet Glob. Health 11 , e655–e657 (2023).
Carbonell-Estrany, X. et al. Identifying the research, advocacy, policy and implementation needs for the prevention and management of respiratory syncytial virus lower respiratory tract infection in low- and middle-income countries. Front Pediatr. 10 , 1033125 (2022).
Srikantiah, P. & Klugman, K. P. New respiratory syncytial virus immunization products in low- and middle-income countries: potential for cost-effective impact on a high burden of disease in young infants. BMC Med. 21 , 177 (2023).
Derksen-Lazet, N. D., Parmentier, C. E. J., Wildenbeest, J. G., Bont, L. J. & Investigators, R. Patient involvement in RSV research: towards patients setting the research agenda. J. Infect. Dis. 226 , S130–S134 (2022).
Langedijk, A. C. et al. A systematic review on global RSV genetic data: Identification of knowledge gaps. Rev. Med. Virol. 32 , e2284 (2022).
Simoes, E. A. F. et al. Suptavumab for the prevention of medically attended respiratory syncytial virus infection in preterm infants. Clin. Infect. Dis. 73 , e4400–e4408 (2021).
Lin, G. L. et al. Distinct patterns of within-host virus populations between two subgroups of human respiratory syncytial virus. Nat. Commun. 12 , 5125 (2021).
Tabor, D. E. et al. Global molecular epidemiology of respiratory syncytial virus from the 2017-2018 INFORM-RSV study. J. Clin. Microbiol. 59 , e01828–20 (2020).
Wilkins, D. et al. Nirsevimab binding-site conservation in respiratory syncytial virus fusion glycoprotein worldwide between 1956 and 2021: an analysis of observational study sequencing data. Lancet Infect. Dis. 23 , 856–866 (2023).
Kim, S. et al. RSV genomic diversity and the development of a globally effective RSV intervention. Vaccine 39 , 2811–2820 (2021).
Salimi, V. et al. Proposal for human respiratory syncytial virus nomenclature below the species level. Emerg. Infect. Dis. 27 , 1–9 (2021).
Dagan, R. et al. The COVID-19 pandemic as an opportunity for unravelling the causative association between respiratory viruses and pneumococcus-associated disease in young children: a prospective study. EBioMedicine 90 , 104493 (2023).
Download references
Acknowledgements
Author would like to thank Barney Graham, Kaitlyn Morabito, Masaru Kanekiyo, and Alexandrine Derrien-Colemyn for helpful edits and discussions. This work was supported by the intramural research program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Author information
Authors and affiliations.
Vaccine Research Center, National Institute of Allergy and Infectious Diseases, NIH, Bethesda, MD, 20892, USA
Tracy J. Ruckwardt
You can also search for this author in PubMed Google Scholar
Contributions
T.J.R. is accountable for the conception, drafting, and final approval of the completed version.
Corresponding author
Correspondence to Tracy J. Ruckwardt .
Ethics declarations
Competing interests.
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ruckwardt, T.J. The road to approved vaccines for respiratory syncytial virus. npj Vaccines 8 , 138 (2023). https://doi.org/10.1038/s41541-023-00734-7
Download citation
Received : 02 June 2023
Accepted : 13 September 2023
Published : 25 September 2023
DOI : https://doi.org/10.1038/s41541-023-00734-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Mucosal bivalent live attenuated vaccine protects against human metapneumovirus and respiratory syncytial virus in mice.
- Daniela Ogonczyk-Makowska
- Pauline Brun
- Julia Dubois
npj Vaccines (2024)
Drug repurposing screen identifies lonafarnib as respiratory syncytial virus fusion protein inhibitor
- Svenja M. Sake
- Xiaoyu Zhang
- Thomas Pietschmann
Nature Communications (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
CDC Updates RSV Vaccination Recommendation for Adults
For the upcoming respiratory virus season, CDC recommends everyone age 75 and older receive the RSV vaccine
For Immediate Release, Wednesday, June 26, 2024 Contact: Media Relations (404) 639-3286 [email protected]
Today, CDC updated its recommendation for the use of Respiratory Syncytial Virus (RSV) vaccines in people ages 60 and older. For this upcoming respiratory virus season, CDC recommends:
- Everyone ages 75 and older receive the RSV vaccine.
- People ages 60–74 who are at increased risk of severe RSV, meaning they have certain chronic medical conditions, such as lung or heart disease, or they live in nursing homes, receive the RSV vaccine.
This recommendation is for adults who did not get an RSV vaccine last year. The RSV vaccine is not currently an annual vaccine, meaning people do not need to get a dose every RSV season. Eligible adults can get an RSV vaccine at any time, but the best time to get vaccinated is in late summer and early fall before RSV usually starts to spread in communities.
Today’s updated recommendation for people 60 and older replaces the recommendation made last year to simplify RSV vaccine decision-making for clinicians and the public.
Immunizations were available last year for the first time to protect people at increased risk for severe RSV, including infants and young children, and people ages 60 and older. Today’s updated recommendation is based on analyses of RSV disease burden among people 60 and older, as well as RSV vaccine effectiveness and cost-effectiveness studies. Those studies included the first real-world data since RSV vaccines were recommended for people 60 and older.
Healthcare providers should recommend RSV vaccines to their eligible patients, as well as discuss what other vaccines they will need this fall to help prevent respiratory infections.
The following is attributable to CDC Director Dr. Mandy Cohen:
“The CDC has updated its RSV vaccination recommendation for older adults to prioritize those at highest risk for serious illness from RSV,” said Mandy Cohen, M.D., M.P.H. “People 75 or older, or between 60-74 with certain chronic health conditions or living in a nursing home should get one dose of the RSV vaccine to provide an extra layer of protection.”
### U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES
Whether diseases start at home or abroad, are curable or preventable, chronic or acute, or from human activity or deliberate attack, CDC’s world-leading experts protect lives and livelihoods, national security and the U.S. economy by providing timely, commonsense information, and rapidly identifying and responding to diseases, including outbreaks and illnesses. CDC drives science, public health research, and data innovation in communities across the country by investing in local initiatives to protect everyone’s health.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

Clinical characteristics and outcome of respiratory syncytial virus infection among adults hospitalized with influenza-like illness in France
1) IAME, UMR 1137, INSERM, Université Paris Diderot, Sorbonne Paris Cité, France
2) Service de Maladies Infectieuses et Tropicales, Hôpital Bichat, AP-HP, Paris, France
3) Inserm, F-CRIN, Innovative clinical research network in vaccinology (I-REIVAC), Paris, France
4) CNR Virus Influenza, Institut des Agents Infectieux, GHN, Hospices Civils de Lyon, Lyon, France
V. Foulongne
5) CHU de Montpellier, Laboratoire de Virologie, Hôpital Saint Eloi, Inserm U 1058, Montpellier, France
6) AP-HP, Hôpital Cochin Broca Hôtel-Dieu, Laboratoire de Virologie, Paris, France
7) AP-HP, Hôpital Bichat-Claude Bernard, Laboratoire de Virologie, Paris, France
8) CHRU Pontchaillou, Laboratoire de Virologie, Rennes, France
9) CHU Dupuytren, Service Bactériologie, Virologie, Hygiène, Limoges Cedex, France
10) CHU Dupuytren, Laboratoire de Virologie, Limoge Cedex, France
11) Inserm CIC 1425, Hôpital Bichat-Claude Bernard, APHP, Paris, France
12) CHU de Montpellier, Inserm CIC 1411, Hôpital Saint-Eloi, Montpellier, France
13) CHU Dupuytren, CIC 1435, Limoge Cedex, France
P. Tattevin
14) CHRU Pontchaillou, Maladies Infectieuses et Réanimation Médicale, CIC INSERM 1414, Rennes, France
15) Service d’Hygiène, Epidémiologie et Prévention, Groupement Hospitalier Edouard Herriot, Lyon, France
16) Emerging Pathogens Laboratory – Fondation Mérieux, Centre International de Recherche en Infectiologie (CIRI) Inserm U1111, CNRS UMR5308, ENS de Lyon, UCBL1, Lyon, France
17) Sorbonne Universités, UPMC Univ Paris 06, INSERM, Institut Pierre Louis d’Epidémiologie et de Santé Publique (IPLESP UMRS 1136), Paris, France
18) Public health department, Hopital Saint-Antoine, APHP, Paris, France
19) CIRI, Centre International de Recherche en Infectiologie, Virpath, Inserm, U1111, Université Claude Bernard Lyon 1, CNRS, UMR5308, École Normale Supérieure de Lyon, Hospices Civils de Lyon, Univ Lyon, Lyon, France
20) Inserm, CIC 1417, Paris, France
21) Université Paris Descartes, Sorbonne Paris Cité, Paris, France
22) Department of Infectious Diseases, CIC Cochin Pasteur, Cochin Broca Hôtel-Dieu hospital, Assistance Publique-Hôpitaux de Paris (AP-HP), Paris, France
Associated Data
The aim of this study was to analyse characteristics and outcome of respiratory syncytial virus (RSV) infection in adults hospitalized with influenza-like illness (ILI).
Patients hospitalized with ILI were included in this prospective, multicentre study carried out in six French hospitals during three consecutive influenza seasons (2012–2015). RSV and other respiratory viruses were detected by multiplex PCR in nasopharyngeal swabs. Risk factors for RSV infection were identified by backward stepwise logistic regression analysis.
A total of 1452 patients hospitalized with ILI were included, of whom 59% (861/1452) were >65 years and 83% (1211/1452) had underlying chronic illnesses. RSV was detected in 4% (59/1452), and influenza virus in 39% (566/1452). Risk factors for RSV infection were cancer (adjusted OR 2.1, 95% CI 1.1–4.1, p 0.04), and immunosuppressive treatment (adjusted OR 2.0, 95% CI 1.1–3.8, p 0.03). Patients with RSV had a median length of stay of 9 days (6–25), and 57% of them (30/53) had complications, including pneumonia (23/53, 44%) and respiratory failure (15/53, 28%). Fifteen per cent (8/53) were admitted to an intensive care unit, and the in-hospital mortality rate was 8% (4/53). Pneumonia was more likely to occur in patients with RSV than in patients with RSV-negative ILI (44% (23/53) versus 26% (362/1393), p 0.006) or with influenza virus infection (44% versus 28% (157/560), p 0.02).
RSV is an infrequent cause of ILI during periods of influenza virus circulation but can cause severe complications in hospitalized adults. Risk factors for RSV detection in adults hospitalized with ILI include cancer and immunosuppressive treatment. Specific immunization and antiviral therapy might benefit patients at risk.
Introduction
Over the past decade, respiratory syncytial virus (RSV) has been increasingly recognized as an important pathogen in adults [1] , and especially the elderly [2] , [3] , [4] , immunocompromised patients [5] , [6] and individuals with underlying chronic respiratory diseases [7] , [8] . In the elderly population, RSV is one of the three most common causes of respiratory disease, along with influenza virus and rhinovirus [3] , [7] .
Respiratory virus detection has improved with the development of highly sensitive and specific molecular methods, which are providing interesting new epidemiological information. However, for reasons of cost and the lack of RSV-specific antiviral drugs, adult inpatients with influenza-like illness (ILI) are frequently not tested for RSV but rather only tested for influenza virus. Influenza surveillance systems based on ILI and acute respiratory illness definitions are unsuitable for capturing cases of RSV in children and adults [9] , [10] , [11] . More studies are needed to determine the burden of RSV infection, and to characterize at-risk populations that could be targeted by vaccination and antiviral treatment. The aims of this study were to describe (a) the prevalence, (b) the clinical features, and (c) the outcome of RSV infection in adults hospitalized with ILI.

Materials and methods
Study design.
We analysed cases of laboratory-confirmed RSV infection during three consecutive influenza seasons (2012/13, 2013/14 and 2014/15), in a post hoc analysis of patients hospitalized with ILI in the FLUVAC study. FLUVAC is a French prospective observational study of influenza vaccine efficacy conducted in six university hospitals (Cochin Hospital, Paris; Bichat Hospital, Paris; Pontchaillou Hospital, Rennes; Limoges Hospital; University Hospital, Montpellier; Edouard Herriot Hospital, Lyon). The FLUVAC study design is further described in Rondy et al. and Loubet et al. [12] , [13] . Each season, enrolments take place during periods of influenza circulation (from November to March). Data on adults hospitalized for at least 24 h for ILI, with symptom onset <7 days before sampling, were collected. ILI was defined as a combination of the following: (a) at least one of the following systemic symptoms: fever (≥38°C), headache, myalgia or malaise, and (b) at least one of the following respiratory symptoms: cough, sore throat or dyspnoea [14] . Each participant was interviewed, and nasopharyngeal samples were obtained at enrolment.
Study population and data collection
We collected demographic characteristics, chronic underlying diseases and their treatments, the characteristics of the current ILI episode: clinical presentation, hospitalization ward, the length of hospital stay and outcome (occurrence of complication, intensive care unit admission and death). Data were retrieved from the medical charts, interviews with the patients and families, and laboratory databases. All variables collected are detailed in the Supplementary material ( Appendix S1 ).
Laboratory data
Respiratory viruses were detected in nasopharyngeal swabs from all the patients by means of multiplex RT-PCR. Any bronchoalveolar lavage fluid samples or tracheal aspirates ordered by the physician in charge were also tested.
Samples were first tested in the virology laboratory of each participating hospital by means of real-time influenza A and B PCR after manual nucleic acid extraction. All samples were then sent to the French National Influenza Reference Centre (CNR-Lyon) for influenza confirmation and screening for other respiratory viruses. RNA and DNA were extracted with the automated Easymag system from BioMérieux (Marcy l’Etoile, France), and influenza viruses were detected with an in-house real-time RT-PCR protocol [15] . The samples were also screened for a panel of other respiratory viruses (adenoviruses, bocaviruses, coronaviruses, human metapneumovirus, parainfluenza viruses 1–4, picornavirus and RSV) by real-time PCR using the Respiratory Multiwell System r-gene ® on an ABI 7300 analyser.
Statistical analysis
We first described the characteristics of all the patients hospitalized with ILI. Then, univariate analysis was used to compare RSV-positive patients with (a) RSV-negative patients (including patients infected by other respiratory viruses and patients free of viral infection) and (b) influenza virus-positive patients. Quantitative variables were expressed as mean and standard deviation (SD) or median and interquartile range (IQR), and qualitative variables as number and percentage. We used the Wilcoxon rank sum test or Fisher’s exact test for univariable comparisons. Missing data for each variable were excluded from the denominator.
Factors associated with RSV infection were identified by using RSV-negative individuals as the comparison group. Individuals with influenza virus and RSV co-infection were excluded from the analysis. We used a backward stepwise logistic regression model, with RSV test results (positive/negative) as the dependent variable. All covariates with a p value <0.2 in univariate analysis were tested in the multivariate model, namely age (considered as a binary variable (<65 and ≥65 years)), dyspnoea, fever, myalgia, chronic lung disease, cancer (solid and haematological malignancies), diabetes, chronic renal failure, and immunosuppressive treatment. Factors associated with pneumonia onset were analysed among all ILI patients. Individuals with influenza virus and RSV co-infection were excluded from the analysis. We used a backward stepwise logistic regression model in which pneumonia (positive/negative) was the dependent variable. Covariates with a p value <0.2 in univariate analysis were tested in the multivariate model, namely age (considered as a continuous variable), chronic heart disease, chronic respiratory disease, cancer (solid and haematological malignancies), diabetes, chronic renal failure, immunosuppressive treatment, RSV infection, influenza virus infection, and influenza vaccination. The final model was adjusted for chronic respiratory disease and age because of their known role in the onset of pneumonia. Results from both regression models were expressed as odds ratios (OR) and adjusted ORs (aOR) with their 95% CI. A p value of ≤0.05 was considered statistically significant. All analyses used Stata software (V12, © Copyright 1996–2014 StataCorp LPt, College Station, TX, USA).
The FLUVAC study (clinicaltrials.gov {"type":"clinical-trial","attrs":{"text":"NCT02027233","term_id":"NCT02027233"}} NCT02027233 ) respected Good Epidemiological and Clinical Practices in Clinical Research, and the Declaration of Helsinki, and was approved by regional ethics committees. All the study participants gave their informed consent for respiratory virus testing.
Characteristics of the patients with ILI, and virus distribution
Overall, 1452 patients hospitalized with ILI were included during the three periods of influenza circulation ( Fig. 1 ). Median age was 70 years (IQR 54–82), 83% of patients (1211/1452) had at least one chronic underlying disease (mainly chronic respiratory disease or chronic heart disease), 46% of patients (661/1452) had already been hospitalized in the previous 12 months, and 45% of patients (644/1431) had been vaccinated against influenza in the respective season.

Study flowchart.
Among the 1452 patients tested, 777 patients (777/1452, 54%) were positive for at least one respiratory virus. Fifty-nine cases of RSV infection were detected in total (4% (59/1452) of patients with ILI, 8% (59/777) of patients with at least one respiratory virus), 21 in 2012/13, 13 in 2013/14 and 25 in 2014/15 ( Table 1 , Fig. 2 ).
Table 1
Number and percentage of patients hospitalized with influenza-like illness who tested positive for respiratory syncytial virus (RSV), influenza virus or any respiratory virus

Monthly distribution of respiratory syncytial virus infections in the FLUVAC study.
Respiratory syncytial virus was the third most frequent virus, after influenza virus (39% (566/1452) of patients with ILI, 73% (566/777) of patients with at least one virus) and picornavirus (5% (68/1452) of patients with ILI, 9% (68/777) of patients with at least one virus). The other detected viruses were coronavirus (3.5% (51/1452) and 7% (51/777) respectively), human metapneumovirus (3% (41/1452) and 5% (41/777)), adenovirus (1% (18/1452) and 2% (18/777)) and bocavirus (0.5% (8/1452) and 1% (8/777)). Six patients (6/1452, 0.4%) were diagnosed with both influenza virus and RSV infection and so were removed from further analyses.
Characteristics and outcome of RSV-positive patients
The median age of the 53 patients with RSV infection alone was 74 years (IQR, 61–84) ( Table 2 ). Chronic underlying diseases were present in 45 cases (45/53, 85%), and consisted mainly of chronic respiratory diseases (29/53, 55%), chronic heart disease (24/53, 45%) and cancer (18/53, 34%; 12 solid tumours, 6 haematological malignancies). Fifteen patients (15/53, 28%) were on immunosuppressive therapy. Twenty-six patients (26/53, 49%) had been hospitalized in the previous year, an average of 1.4 times (SD 2.3).
Table 2
Characteristics, clinical presentation and outcome of patients hospitalized with influenza virus or respiratory syncytial virus (RSV) infection between 2012 and 2015 in six French university hospitals (excluding patients infected with both RSV and influenza virus).
Abbreviations: BMI, body mass index; IQR, interquartile range
The median time from symptom onset to admission was 2 days (IQR, 1–3). Cough (43/53, 81%), fever (44/53, 83%) and dyspnoea (45/53, 85%) were the main symptoms in patients with RSV infection.
The median length of hospital stay was 9 days (IQR 6–25). A total of 55 medical complications occurred in 30 patients (30/52, 58%) during their hospital stay, including pneumonia (23 episodes, 42% (23/55) of complications), respiratory failure (15/55, 27%), heart failure (10/55, 18%), and acute respiratory distress syndrome (7/55, 13%). Intensive care unit admission was necessary for eight patients (8/53, 15%). Four patients (4/53, 8%) died during the hospital stay; all of them were men >65 years old with chronic respiratory diseases. Furthermore, three patients had chronic heart disease. The mean time from admission to death was 13 days (SD 2.9).
RSV-positive patients versus RSV-negative patients and influenza virus-positive patients
Patients with RSV were older than patients with influenza virus (74 years (IQR 1–84) versus 68 years (IQR 52–81), p 0.05) ( Table 2 ).
Patients with RSV were more likely to have cancer or immunosuppressive treatment than patients without RSV (respectively 34% (18/53) versus 16% (219/1393), p 0.002; 28% (15/53) versus 15% (205/1393), p 0.003) and influenza virus-positive patients (34% (18/53) versus 13% (74/560), p 0.004; 28% (15/53) versus 14% (78/560), p 0.01).
Multivariate analysis of all patients with ILI showed that cancer (OR 2.1; 95% CI 1.1–4.1, p 0.04) and immunosuppressive treatment (OR 2.0; 95% CI 1.1–3.8, p 0.03) were significantly associated with RSV detection ( Table 3 ).
Table 3
Factors associated with respiratory syncytial virus (RSV) infection in multivariable logistic regression (excluding patients infected with both RSV and influenza virus)
The outcome analysed was RSV infection.
The patients with RSV and influenza virus infection did not differ in terms of the length of stay, ICU admission or mortality.
After adjustment for chronic respiratory disease and age, we found that RSV infection (OR 2.1; 95% CI 1.2–3.8, p 0.008), chronic renal failure (OR 1.8; 95% CI 1.3–2.5, p 0.001) and active smoking (OR 1.3; 95% CI 1.0–1.7, p 0.02) were significantly associated with the onset of pneumonia ( Table 4 ).
Table 4
Factors associated with pneumonia occurrence ( n = 385) among hospitalized patients with influenza-like illness in multivariable logistic regression (excluding patients infected with both respiratory syncytial virus (RSV) and influenza virus)
The outcome analysed was the occurrence of pneumonia during the hospital stay ( n = 385).
The multivariate model was adjusted for 'chronic respiratory disease' and 'age >65'.
In this study conducted during three consecutive influenza seasons among 1452 adults hospitalized for ILI in France, RSV was the third most common respiratory virus, being detected in 4% of patients, compared with 39% for influenza virus. This rate is lower than that found in the USA by Sundaram et al. in a community-based cohort of adults aged >50 years during six influenza seasons (184/2225, 8%) [3] , or by Falsey et al. among community-based elderly individuals from 14 countries with moderate to severe ILI (41/556, 7%) [7] . In the latter study, the rate was even higher (8/64, 12.5%) among hospitalized patients. In another study, Falsey et al. reported a 10% (142/1388) prevalence of RSV among adults aged ≥65 years or with underlying cardiopulmonary diseases who were admitted with acute respiratory symptoms during four consecutive winters in Rochester, NY [1] . Several reasons may explain the broad range of reported RSV detection rates. For example, our study was restricted to periods of influenza virus circulation, which may not have included the peak of RSV circulation, although enrolments in the two studies by Falsey et al. started in mid-November. Another factor is age: we included all adults >18 years, whereas Sundaram et al. and Falsey et al. included only older subjects, in whom RSV infection may be more frequent. Furthermore, the laboratory methods used in the Rochester study included viral culture and serological tests, and RT-PCR was the only positive test in only two-thirds of cases. Finally, the clinical definition used to trigger swab collection also differed among the studies.
The median age of the RSV-infected patients in our study was 74 years but, contrary to other studies, we found no association between RSV infection and older age [3] , [16] , [17] . Most of the patients with RSV infection had chronic underlying conditions (45/53, 85%), consisting mainly of chronic respiratory disease (29/53, 55%) or chronic heart disease (24/53, 45%). This is consistent with the report by Walsh et al. that underlying pulmonary disease was a significant risk factor for severe RSV illness requiring hospitalization [18] .
We found that the two underlying conditions independently associated with RSV infection were cancer and immunosuppressive treatment. This association was statistically significant whether RSV-positive patients were compared with all RSV-negative patients or only with influenza virus-positive patients. Although immunocompromised patients (especially patients with haematological malignancies) are known to be more susceptible to RSV infection [19] , solid cancers and immunosuppressive therapy are not classical risk factors.
Respiratory syncytial virus was associated with significant morbidity: the median length of hospital stay was 9 days; 15% (8/53) of RSV-infected patients were admitted to the intensive care unit, and 8% (4/53) died. These findings are consistent with the literature [1] , [20] . Similar percentages were noted in the influenza group of our study. Patients with RSV were significantly more likely than patients with influenza or without RSV to develop pneumonia (44% versus 28% and 26%, respectively). Falsey et al. and Lee et al. also found a high rate of pneumonia among 159 RSV-infected patients >65 years admitted for ILI in the USA (70/159, 44%) and among 607 RSV-infected adults admitted to three hospitals in Hong Kong (261/603, 43%) [20] , [21] . Jain et al. [22] found at least one respiratory virus in 23% of 2259 American adults with community-acquired pneumonia requiring admission to one of five participating hospitals between 2010 and 2012. RSV was found in 3% (67/2259) of all patients.
The strengths of this study include the large number of adults hospitalized with ILI, the prospective multicentre design, standardized patient screening in the participating centres, centralized confirmation of respiratory viruses in an influenza reference centre and the lengthy study period spanning three consecutive influenza seasons. Several limitations must, however, be acknowledged. First, as all the participating centres were teaching hospitals, the proportion of patients with underlying diseases may have been higher than in the general ILI population, owing to a referral bias. Second, although the sample was large, the study was probably underpowered to identify a possible impact of RSV on morbidity and mortality in multivariate analysis. Third, the ILI definition used here captures only a subset of RSV infections [10] . Our study does not reflect the real burden of severe RSV infection, which may include other clinical manifestations. Fourth, we report post hoc results of the FLUVAC study, which was not designed to answer this research question. Indeed, as the FLUVAC study was designed to assess influenza vaccine efficacy, patients were enrolled during periods of influenza virus circulation, which differ slightly from periods of RSV circulation. This means that our data reflect the prevalence of RSV among patients hospitalized for ILI during periods of influenza virus circulation and not during peak RSV circulation, which usually occurs earlier, owing to epidemiological interference [23] . However, French surveillance data (RENAL system) show that the peak of the RSV epidemic overlapped with the beginning of influenza virus circulation during the three seasons of interest, and that the two viruses co-circulated for at least 2–3 weeks ( Fig. 3 ).

Respiratory syncytial virus and influenza virus circulation in France during the 2012/13 (a), 2013/14 (b) and 2014/15 (c) seasons (data from the GROG, RENAL and Sentinelles surveillance networks) (arrows correspond to the start and end of fluvac study period).
In conclusion, this prospective observational study conducted in France during three influenza seasons reveals that 4% of adults hospitalized for ILI had RSV infection, of whom 58% developed cardiopulmonary complications and 8% died. It also shows that elderly individuals and patients with cancer and/or immunosuppressive treatment are more likely to have RSV isolated when hospitalized for ILI. Potential benefits of enhanced RSV testing, antiviral treatment, and vaccine development in these groups should be considered.
The current work received no funding. However, the study sites received funding from Sanofi Pasteur and Sanofi Pasteur MSD for the FLUVAC study. Vaccine producers had no role in the study design, data analysis, decision to publish or preparation of the manuscript.
Transparency declaration
The authors declare no competing interest related to the study. O Launay is an investigator for clinical trials sponsored by Janssen and other companies and received travel support to attend scientific meetings from pharmaceutical companies.
Acknowledgements
We thank all the RENAL network laboratories and the CNR laboratory for agreeing to share their data on influenza and RSV epidemics. We specifically thank Vincent ENOUF (CNR Influenza, Paris) for creation of Fig. 3 .
We are very grateful to the following persons and institutions who made significant contributions to the FLUVAC study: Réseau National d’Investigation Clinique en Vaccinologie (I-REIVAC): K. Seddik, Z. Lesieur, N. Lenzi; InVS, France: I. Bonmarin; FLUVAC Study group: Hôpital Cochin, Paris: O. Launay, P. Loulergue, H. Bodilis, M. Servera-Miyalou, I. Sadler, S. Momcilovic, R. Kanaan, N. Coolent, K. Tan Boun, P. Blanche, J. Charpentier, F. Daviaud, N. Mongardon, A. Bretagnol, YE. Claessens, F. Rozenberg, A. Krivine. Hôpital Bichat Claude-Bernard, Paris: Y. Yazdanpanah, C. Burdet, S. Harent, M. Lachatre, C. Rioux, A. Bleibtreu, E. Casalino, C. Choquet, A. Leleu, K. Belghalem, L. Colosi, M. Ranaivoson, V. Verry, L. Pereira, E. Dupeyrat, J. Bernard, N. Emeyrat, P. Chavance, A. Debit, M. Aubier, P. Pradere, A. Justet, H. Mal, O. Brugiere, T. Papo, T Goulenok, M. Boisseau, R. Jouenne, JF. Alexandra, A. Raynaud-Simon, M. Lilamand, A. Cloppet-Fontaine, K. Becheur, AL. Pelletier, N. Fidouh, P. Ralaimazava, F. Beaumale, Y. Costa, X. Duval. Hôpital Edouard Herriot, Lyon: E. Munier, F. Betend, S. Amour, S. Loeffert, K. Francourt. Hôpital Saint-Eloi, Montpellier: C. Merle, F. Galtier, F. Letois, V. Foulongne, P. Géraud, V. Driss, S. Noslier, M. Ray, M. Sebbane, A. Konaté, A. Bourdin, K Klouche, M.S. Léglise. CHU Dupuytren, Limoges: D. Postil, E. Couve-Deacon, D. Fruit, C. Fenerol, C. Vallejo, S. Alain, S. Rogez; CHU Pontchaillou, Rennes: P. Tattevin, S. Jouneau, F. Lainé, E. Thébault, G. Lagathu, P. Fillatre, C. Le Pape, L. Beuzit; Institut Pierre Louis d’Epidémiologie et de Santé Publique (IPLESP UMRS 1136): F. Carrat, F. Chau, I. Goderel et de Santé Publique (IPLESP UMRS 1136): F. Carrat, F. Chau, I. Goderel.
The authors thank Hélène Bricout, Laurence Pagnon and Christine Sadorge from Sanofi Pasteur MSD for their inputs during the study design phase and for critical review of the results.
Editor: C. Pulcini
Appendix A Additional Supporting Information may be found in the online version of this article at http://dx.doi.org/10.1016/j.cmi.2016.11.014 .
Contributor Information
23 Réseau National d’Investigation Clinique en Vaccinologie (I-REIVAC), France
I. Bonmarin
24 InVS, France
P. Loulergue
25 FLUVAC Study group: Hôpital Cochin, Paris, France
M. Servera-Miyalou
S. momcilovic, k. tan boun, j. charpentier, n. mongardon, a. bretagnol, y.e. claessens, f. rozenberg, y. yazdanpanah.
26 Hôpital Bichat Claude-Bernard, Paris, France
M. Lachatre
A. bleibtreu, e. casalino, k. belghalem, m. ranaivoson, e. dupeyrat, p. chavance, o. brugiere, t. goulenok, m. boisseau, j.f. alexandra, a. raynaud-simon, m. lilamand, a. cloppet-fontaine, a.l. pelletier, p. ralaimazava, f. beaumale.
27 Hôpital Edouard Herriot, Lyon, France
S. Loeffert
K. francourt.
28 Hôpital Saint-Eloi, Montpellier, France
P. Géraud
A. konaté, m.s. léglise, e. couve-deacon.
29 CHU Dupuytren, Limoges, France
30 CHU Pontchaillou, Rennes, France
F. Lainé
E. thébault, p. fillatre.
40 Institut Pierre Louis d’Epidémiologie et de Santé Publique (IPLESP UMRS 1136), France
41 I. Goderel et de Santé Publique (IPLESP UMRS 1136), France
Appendix A. Supporting information
The following supplementary materials are available for this article:
Appendix S1 .
Language selection
- Français fr
Respiratory syncytial virus (RSV) vaccines: Canadian Immunization Guide
For health professionals
- Part 4 table of contents
This chapter was updated based on the following guidance from the National Advisory Committee on Immunization (NACI):
- August 9, 2024: Statement on the prevention of respiratory syncytial virus (RSV) disease in older adults
On this page
Key information.
List 1: Infants at increased risk of severe RSV disease
List 2: Chronic health conditions in older adults that lead to increased risk for severe RSV disease
Preparations authorized for use in Canada
Immunogenicity, efficacy and effectiveness, routine schedule, additional doses, infants born prematurely, infants and children with chronic diseases, infants and children who are immunocompromised, infants and children whose transportation for severe rsv disease treatment is complex, infants and children whose risk of severe rsv disease intersects with established social and structural health determinants, pregnant or breastfeeding women and pregnant or breastfeeding people, older adults, administration practices, storage requirements, safety and adverse events, chapter revision process, acknowledgments, selected references.
Please note: The Public Health Agency of Canada (PHAC) recognizes that not all people giving birth or breastfeeding will identify as women or mothers. The writing in this chapter uses a gender additive approach where the term 'woman' is used alongside gender neutral language. This is intended to demonstrate a commitment to redress the historic exclusion of trans and non-binary people, whilst avoiding the risk of marginalizing or erasing the experience of women within the health care environment. The dynamic nature of language is recognized. It is likely that language deemed to be suitable or affirming in one context may not translate across others, and over the coming years will continue to change and evolve with respect to appropriate representations. In line with best practice, it is recognized that when discussing or caring for individuals in a one-on-one capacity language and documentation should reflect the gender identity of the individual.
- Respiratory syncytial virus (RSV) infection is a major cause of lower respiratory tract illness, particularly among infants, young children and older adults.
- In Canada, RSV causes yearly outbreaks of respiratory tract disease, usually starting in late fall and running through to early spring.
- Reinfections with RSV are common but illness is usually milder with subsequent infections until older adulthood, when RSV can again lead to severe disease.
- Immunization products to protect infants against RSV include: two monoclonal antibody preparations, nirsevimab and palivizumab which are administered directly to infants, as well as the RSVpreF vaccine (Abrysvo TM ), which is administered during pregnancy to protect the infant through the passive transfer of maternal antibodies.
- Two vaccines are available to protect adults 60 years of age and older from RSV; RSVPreF3 (Arexvy) and RSVpreF (Abrysvo TM ).
- RSV immunization should be considered for any infant entering, or born during, their first RSV season and for infants at ongoing increased risk during their second RSV season.
- Nirsevimab, the RSV monoclonal antibody, is preferred over palivizumab and the RSVpreF (Abrysvo TM ) vaccine.
- Nirsevimab should be prioritized for infants:
- With certain medical conditions that place them at increased risk of severe RSV disease including prematurity (List 1)
- With certain medical conditions (List 1) who are at ongoing increased risk for severe RSV disease during their second RSV season
- Whose transportation for severe RSV disease treatment is complex, and/or who live in or are part of First Nations, Métis, and Inuit communities where risk of severe RSV disease intersects with established social and structural health determinants.
- If nirsevimab is not available, palivizumab may be used for certain infants at very high risk for severe RSV disease. For more information refer to sections: Infants born prematurely , Infants and children with chronic diseases , Infants and children whose transportation for severe RSV disease treatment is complex , and Infants and children whose risk of severe RSV disease intersects with established social and structural health determinants .
- The RSVpreF (Abrysvo TM ) vaccine may be considered by a pregnant woman or pregnant person, together with their care provider, in advance of, or during, the RSV season, to prevent RSV disease in their infant. There is no expected additional benefit to using both RSVpreF (Abrysvo TM ) and nirsevimab for healthy infants. However, if RSVpreF (Abrysvo TM ) was received during pregnancy and the infant is at increased risk for severe RSV disease or is born less than 2 weeks after RSVpreF (Abrysvo TM ) was given, nirsevimab should still be provided.
- 75 years of age and older, particularly for those who are at increased risk of severe RSV disease ( List 2 )
- 60 years of age and older who are residents of nursing homes and other chronic care facilities.
- RSV immunization may be considered as an individual decision for adults 60 to 74 years of age in consultation with their health care provider
- RSV immunizing products are optimally administered just before the start of the RSV season. Jurisdictions should define their RSV season based on local epidemiology to determine when to provide RSV immunizing products.
- The schedule and dosing of nirsevimab and palivizumab given to infants entering or born during the RSV season are as follows:
- A single dose of nirsevimab is administered to infants. Those weighing less than 5 kg should receive a 0.5 mL dose (50 mg/0.5 mL) and those weighing 5 kg or more should receive a 1 mL dose (100 mg/1 mL).
- For a second RSV season, nirsevimab is given as a single dose of 200 mg (2 x 100 mg/1 mL). However, if the child weighs less than 10 kg, a single dose of 100 mg may be considered at clinical discretion.
- For palivizumab, a series of 4 or 5 doses, each of 15 mg/kg of body weight, are administered approximately 28 days apart throughout the RSV season.
- A second dose of palivizumab should follow at 21 to 28 days and the interval between subsequent doses is 28 to 35 days.
- An additional dose should be given after cardiac bypass or extracorporeal membrane oxygenation.
- An additional dose may be considered in remote northern areas where RSV outbreaks may continue longer than is usual elsewhere.
- A 0.5 mL dose of RSVpreF (Abrysvo TM ) vaccine is administered during pregnancy between 32 and 36 weeks gestation.
- The protective efficacy of RSVpreF (Abrysvo TM ) takes some time to develop; therefore, it is optimally administered at least 2 weeks before birth to allow for the transplacental transfer of protective antibodies.
- A single 0.5 mL dose of RSVPreF3 (Arexvy) or RSVpreF (Abrysvo TM ) is administered intramuscularly in adults 60 years of age and older.
- RSV is the most common cause of bronchiolitis and pneumonia among infants and young children.
- RSV disease can have serious complications for infants, including hospitalization and intensive care unit admission, as well as significant impact on their caregivers and families.
- RSV accounts for a significant burden of disease in older adults, particularly those with certain chronic health conditions ( List 2 ), and can result in serious complications, including hospitalization, intensive care unit (ICU) admission and death.
- Immunization of older adults with RSV vaccine can reduce medically attended RSV respiratory tract infections and hospitalizations due to RSV.
- The RSV monoclonal antibodies, nirsevimab and palivizumab, along with the RSVpreF (Abrysvo TM ) vaccine can help protect infants from RSV disease by giving the infant antibodies, either via direct injection or transplacental transfer.
- Reducing severe outcomes from RSV in infants and older adults at the population level may help to protect health system capacity.
Epidemiology
Disease description, infectious agent.
RSV is an enveloped single-stranded RNA virus from the Paramyxoviridae family. RSV is a common cause of respiratory tract infections that recur throughout life. It is the most common cause of bronchiolitis and pneumonia among infants and young children, and is responsible for more severe clinical outcomes among older adults, particularly among those with comorbidities. For additional information about the RSV virus, refer to the Pathogen Safety Data Sheet .
Humans are the only natural reservoir.
Transmission
RSV is transmitted by direct and indirect contact with infectious respiratory tract secretions. Transmission occurs directly when droplets generated from coughs or sneezes of an infected person come into contact with the mucous membranes of the eyes, nose, mouth, or airway of another person. Indirect transmission occurs when people touch contaminated hands, surfaces and objects and inoculate themselves by touching their mucous membranes.
Risk factors
RSV infects almost all infants by 2 years of age. In infants and young children, the younger the child, the higher the risk of hospitalization. The risk of medically attended RSV appears to be higher in infants with comorbidities compared to healthy term infants entering their first RSV season. However, the majority of infants and young children who require medical office or emergency department visits associated with RSV are born at term with no underlying comorbidities.
Prematurity is a notable risk factor for RSV hospitalization. Indeed, infants born at less than 30 weeks gestational age (wGA) have RSV hospitalization rates of 7.7 to 13.6% in the first year of life. Also at higher risk of RSV hospitalization are young children with chronic respiratory, cardiac or immunocompromising conditions. List 1 describes medical conditions that increase an infant's risk of severe RSV disease.
Infants at increased risk of severe RSV disease during their first RSV season:
- All premature infants (i.e., born at less than 37 wGA)
- Chronic lung disease, including bronchopulmonary dysplasia, requiring ongoing assisted ventilation, oxygen therapy or chronic medical therapy in the 6 months prior to the start of the RSV season
- Cystic fibrosis with respiratory involvement and/or growth delay
- Haemodynamically significant chronic cardiac disease
- Severe immunodeficiency Footnote 1 Footnote 2
- Severe congenital airway anomalies impairing clearing of respiratory secretions
- Neuromuscular disease impairing clearing of respiratory secretions
Down syndrome
- All those listed above, except for infants born at less than 37 wGA and infants with Down syndrome who do not have another medical condition on the list.
For a list of immunocompromising conditions, refer to the COVID-19 vaccines chapter
Return to footnote 1 referrer
Criteria for severe immunodeficiency with HIV: CD4 less than 750 cells/µL if age less than 1 year or CD4 less than 500 if age 1 to 2 years
Return to footnote 2 referrer
There is a higher burden of RSV hospital admissions in northern and remote settings compared to the rest of Canada. High rates of RSV hospitalization, particularly among those less than 1 year of age, have been observed among infants in the Yukon, Northwest Territories, and Nunavut as well as in Nunavik, the northernmost part of Quebec.
Adults 75 years of age and older, particularly those with certain chronic health conditions ( List 2 ) are at increased risk of severe RSV disease, hospitalization, intensive care unit (ICU) admission and death. Serious outcomes of RSV infection are also seen in adults 60 years of age and older who are residents of nursing homes and other chronic care facilities. In addition, adults may be at increased risk of severe RSV disease due to factors that intersect with social determinants of health.
- Cardiac or pulmonary disorders (includes chronic obstructive pulmonary disease [COPD], asthma, cystic fibrosis, and conditions affecting ability to clear airway secretions)
- Diabetes mellitus and other metabolic diseases
- Moderate and severe immunodeficiency (refer to the list of immunocompromising conditions developed for COVID-19 )
- Chronic renal disease
- Chronic liver disease
- Neurologic or neurodevelopmental conditions (includes neuromuscular, neurovascular, neurodegenerative [e.g., dementia], neurodevelopmental conditions, and seizure disorders, but excludes migraines and psychiatric conditions without neurological conditions)
- Class 3 obesity (defined as BMI of 40 kg/m 2 and over).
Seasonal and temporal patterns
RSV exhibits a seasonal infection cycle that is somewhat variable by region. In temperate climates in the Northern Hemisphere, annual RSV outbreaks begin in the fall and continue to early spring. Prior to the COVID-19 pandemic, the RSV season in most of Canada was typically November to April. Information on current RSV activity in Canada can be found on the respiratory virus detections in Canada website.
Spectrum of clinical illness
Most RSV infections are respiratory tract infections that present as nasal congestion, cough, low grade fever and loss of appetite and last approximately 1 to 2 weeks. Approximately 20 to 30% of infected infants develop bronchiolitis or pneumonia. Croup or otitis media may also occur. Recurrent infections occur throughout life, with subsequent infections usually less severe than the first until older adulthood, when RSV infection can again lead to severe disease. Older children and most adults usually present with symptoms similar to the common cold. Severe pneumonia may occur in older adults, particularly among those with certain chronic medical conditions ( List 2 ) and in immunocompromised individuals of any age.
Mortality is very low (6.9 per 1 million live births) in high income countries among children receiving supportive care. Mortality is more common among older adults hospitalized for RSV, at approximately 5 to 10% and increases with increasing age and presence of comorbidities.
Disease distribution
RSV occurs worldwide, with virtually all children infected by age two. Globally, RSV is an important cause of acute lower respiratory tract infection and a major cause of hospital admissions in young children, for whom it has been estimated that RSV is associated with about 31% of pneumonia cases and 33 million episodes of acute lower respiratory tract infections. In Canada, approximately 2% of all infants are hospitalized with RSV in their first year of life. In some remote communities, RSV hospitalization rates have been as high as 5 to 17% of all live births. RSV is not a notifiable disease in Canada, consequently the burden and risk of RSV in older adults is likely underestimated.
Monoclonal antibodies
- SYNAGIS ® (palivizumab) is a passive immunizing agent (humanized monoclonal antibody). AstraZeneca Canada Inc.
- BEYFORTUS TM (nirsevimab) is a passive immunizing agent (human monoclonal antibody). Sanofi Pasteur Ltd.
RSV vaccines
- AREXVY (RSVPreF3) RSV subunit adjuvanted vaccine, GlaxoSmithKline Inc.
- ABRYSVO TM (RSVpreF) RSV subunit vaccine, Pfizer Canada.
For complete prescribing information, consult the product leaflets or information contained within Health Canada's authorized product monographs available through the Drug Product Database . Refer to Table 1 in Contents of immunizing agents authorized for use in Canada in Part 1 for a list of all vaccines authorized for use in Canada and their contents.
Nirsevimab has been shown to reduce hospital admission associated with RSV by 81 to 83%. It also has shown an 80% reduction in medically attended RSV respiratory tract infection in healthy infants. Both nirsevimab and palivizumab show efficacy in reducing the rate of medically attended RSV infections in infants at increased risk for severe RSV due to prematurity, congenital heart disease, and chronic lung disease, in their first and second RSV seasons.
The protective efficacy of monoclonal antibodies takes effect immediately. Nirsevimab has a longer durability of protection and, if administered at birth, protection is high in the first months of life when infants are most at risk for RSV. Nirsevimab is efficacious through 5 months of age and may provide full-season protection. Palivizumab has a shorter durability of protection compared to nirsevimab. Among all infants at risk of severe RSV infection, palivizumab prophylaxis is associated with risk reductions of approximately 38 to 86% for RSV-associated hospital admissions. In infants born at 32 wGA or earlier palivizumab was shown to reduce the risk of all-cause mortality.
Infants living in some remote northern communities are at very high risk of hospitalization for RSV. Data on effectiveness of RSV monoclonal antibodies to prevent hospitalization in this group are very limited.
RSVpreF (Abrysvo TM ) and RSVPreF3 (Arexvy) vaccines
RSVpreF vaccine administered to pregnant women and pregnant people results in a reduction in RSV associated hospital admission in their infants by 57%. It also reduces medically attended RSV respiratory tract infection in their infants by 51% in their first RSV season. The protective efficacy of RSVpreF takes some time to develop; therefore, it is optimally administered at least 2 weeks before birth to allow for the transplacental transfer of protective antibodies. Efficacy of RSVpreF vaccine is high in the first months of life when infants are most at risk for RSV during the RSV season. Due to waning of the passively transferred antibodies in neonates over time, the protective effect may not exceed 6 months of age in infants.
The efficacy of RSVpreF vaccine in preventing RSV infection in pregnant women and pregnant people has not been evaluated.
Data are limited, however RSVpreF and RSVPreF3 appear to result in similar reductions in laboratory confirmed RSV respiratory tract infections (RTI) associated hospitalizations and medically attended RSV RTI for adults 60 years of age and older. The efficacy of these vaccines beyond season 1 is not yet clear. However, early data suggest that through two RSV seasons, efficacy against RSV disease may be maintained. NACI will continue to monitor emerging evidence as it becomes available.
The immune response to RSV vaccines wanes after the first dose. The importance of this is not yet clear as there is no established immune correlate of protection.
Recommendations for use
If available, a dose of the RSV monoclonal antibody nirsevimab is recommended for all infants entering, or born during, their first RSV season. Supply may be prioritized for infants and children at increased risk .
For adults 60 years of age and older, one dose of either RSVpreF or RSVPreF3 is optimally administered just before the start of the RSV season.
Infants and children at increased risk
RSV monoclonal antibodies, are recommended for infants and children at increased risk of severe RSV disease (List 1) . Nirsevimab is preferred over palivizumab.
A dose of nirsevimab should be prioritized for infants and children:
- Entering, or born during, their first RSV season who are at increased risk of severe RSV disease, including those who are born at less than 37 wGA (List 1)
- Entering their second RSV season and at ongoing increased risk of severe RSV disease (List 1)
- For infants entering, or born during, their first RSV season whose transportation for severe RSV disease treatment is complex, and/or whose risk of severe RSV disease intersects with established social and structural health determinants such as those experienced by some Indigenous communities across First Nations, Métis and Inuit populations Footnote 1 .
If nirsevimab is not available, palivizumab should be used according to the NACI Statement on the Recommended use of palivizumab to reduce complications of respiratory syncytial virus infection in infants (2022).
Palivizumab is given as 4 or 5 doses in a season. The interval between the first and second doses is 21 to 28 days, and the interval between subsequent doses is 28 to 35 days. The first dose of palivizumab is given at the onset of the RSV season, as determined by local epidemiology.
Infants born during the RSV season and who qualify for palivizumab prophylaxis should receive their first dose 48 to 72 hours before discharge home if possible, or promptly after discharge. Administering the first dose before discharge avoids the need to visit a health care facility soon after discharge and may improve adherence.
An infant who has already begun palivizumab prophylaxis earlier in the season and is re-hospitalized on a date when a dose is due should receive that dose as scheduled while in hospital, providing the admitting institution is able to supply palivizumab when due. Keeping to the child's existing schedule avoids the need to reschedule appointments and may improve adherence.
Monoclonal antibodies (nirsevimab and palivizumab) are not recommended for an infant who has a current confirmed RSV infection or a previous confirmed RSV infection in the current RSV season. Reported rates of second episodes of RSV hospitalization in the same season are very low. Consideration may be given in the case of severely immunocompromised infants who may still benefit from the monoclonal antibodies as they may not mount an immune response to the RSV infection.
Infants whose gestational parent received RSVpreF vaccine do not need nirsevimab unless the infant meets the medical criteria for increased risk of severe RSV disease (List 1) or the infant is born less than 2 weeks after administration of RSVpreF.
Pregnant women and pregnant people
For the prevention of severe RSV disease in infants, nirsevimab administered to the infant is recommended over RSVpreF vaccine administered to the pregnant woman or pregnant person due to current evidence of nirsevimab's superior efficacy, duration of protection, and available safety data.
The RSVpreF vaccine may be considered by a pregnant woman or pregnant person together with their care provider, in advance of, or during the RSV season to prevent severe RSV disease in their infant. Use of RSVpreF vaccine is not required if it is anticipated that nirsevimab will be administered to the infant.
RSVpreF vaccine administration should occur between 32 and 36 weeks gestation and in advance of, or during, the RSV season, to protect infants expected to be born during the RSV season as determined by local RSV epidemiology, to allow the development of a humoral immune response and passive antibody transfer. If RSVpreF is not received at least 2 weeks before birth, there would be insufficient time to allow for the transplacental transfer of protective antibodies.
RSVpreF may be administered regardless of past RSV infection.
An additional dose of monoclonal antibody is recommended after surgery requiring cardiopulmonary bypass and can be considered at the conclusion of extracorporeal membrane oxygenation. Refer to the product monograph for dosing guidance.
The need for a subsequent RSVpreF and RSVPreF3 vaccine dose for adults 60 years of age and older and an optimal strategy for boosting these vaccine responses are not yet clear. NACI will continue to monitor emerging evidence as it becomes available.
Vaccination of specific populations
To prevent severe RSV disease during their first RSV season, RSV monoclonal antibodies are recommended for infants born prematurely. Nirsevimab is preferred over palivizumab and should be provided to infants born at less than 37 wGA and entering, or born during, their first RSV season. Infants and children born at less than 37 wGA who do not have another medical condition (List 1) and are entering their second RSV season are not thought to be at increased risk of severe RSV disease; therefore, they should not be routinely offered monoclonal antibodies for their second RSV season.
If nirsevimab is not available, palivizumab should be used according to the NACI Statement on the Recommended use of palivizumab to reduce complications of respiratory syncytial virus infection in infants (2022), for infants born at less than 33 weeks gestation.
Palivizumab may be considered for infants born at 30 to less than 33 weeks gestation and aged less than 3 months at the onset of or during the RSV season if they are at high risk of exposure to RSV from day care attendance or presence of another preschool child or children in the home.
Palivizumab is not recommended for healthy premature infants born at or after 33 weeks gestation or for infants or siblings of multiple births who do not otherwise have an indication for prophylaxis.
Refer to Immunization of infants born prematurely in Part 3 for additional information about vaccination of premature infants.
Chronic lung disease
To prevent severe RSV disease during their first and second RSV seasons, RSV monoclonal antibodies are recommended for infants and children with chronic lung disease ( List 1 ). Nirsevimab is preferred over palivizumab and should be provided for infants and children with chronic lung disease (including bronchopulmonary dysplasia) requiring ongoing assisted ventilation, oxygen therapy or chronic medical therapy in the 6 months prior to the start of the RSV season, as well as infants and children with cystic fibrosis with respiratory involvement and/or growth delay.
If nirsevimab is not available, palivizumab should be used according to the NACI Statement on the Recommended use of palivizumab to reduce complications of respiratory syncytial virus infection in infants (2022) as follows: Palivizumab is recommended for all infants with chronic lung disease of prematurity (infants born at less than or equal to 32 weeks gestational age and with need for supplemental oxygen (O2) greater than 21% for at least the first 28 days after birth) who are less than 24 months of age at the onset of the RSV season and have required ongoing supplemental O2 therapy or assisted ventilation in the 6 months prior to the onset of or during the RSV season.
Palivizumab may be considered for infants less than 24 months of age with severe chronic lung disease of other etiology (e.g., congenital cystic lung disease, chronic interstitial lung disease, congenital lung malformations, congenital airway abnormalities or neuromuscular conditions affecting ability to clear airway secretions) or who require home respiratory support (e.g., supplemental O2, mechanical ventilation, continuous positive airway pressure, tracheostomy) if requiring ongoing supplemental O2 or assisted ventilation in the 6 months prior to the onset of or during the RSV season.
Palivizumab is not routinely recommended for children less than 24 months of age with cystic fibrosis; however, palivizumab may be considered for those less than 24 months of age with cystic fibrosis who have severe chronic lung disease as defined by the need for ongoing supplemental O2 in the 6 months prior to the onset of or during the RSV season. Palivizumab is not recommended for the prevention of recurrent wheezing or asthma in the absence of other indications.
Refer to Immunization of persons with chronic diseases in Part 3 for additional information about vaccination of people with chronic diseases.
Heart disease
To prevent severe RSV disease during their first and second RSV seasons, RSV monoclonal antibodies are recommended for infants and children with haemodynamically significant chronic cardiac disease. Nirsevimab is preferred over palivizumab. If nirsevimab is not available, palivizumab should be used according to the NACI Statement on the Recommended use of palivizumab to reduce complications of respiratory syncytial virus infection in infants (2022) for children with heart disease. Reimmunization is indicated for individuals undergoing cardiac surgery with cardiopulmonary bypass as soon as the individual is stable after surgery. Reimmunization can also be considered at the conclusion of extracorporeal membrane oxygenation.
To prevent severe RSV disease during their first RSV season, RSV monoclonal antibodies are recommended for infants with Down syndrome. Nirsevimab is preferred over palivizumab. Infants and children with Down syndrome who do not have another medical condition (List 1) and are entering their second RSV season are not thought to be at increased risk of severe RSV disease; therefore, they should not be routinely offered monoclonal antibodies for their second RSV season.
If nirsevimab is not available, palivizumab should be used according to the NACI Statement on the Recommended use of palivizumab to reduce complications of respiratory syncytial virus infection in infants (2022) as follows: Palivizumab should be offered to children with Down syndrome who qualify for prophylaxis because of haemodynamically significant congenital heart disease, chronic lung disease, prematurity or immunodeficiency, but should not be routinely offered to all children less than 24 months of age with Down syndrome.
In general, people who are immunocompromised are more susceptible to vaccine-preventable infections and may have more severe infections. Monoclonal antibodies are recommended to prevent severe RSV disease during the first and second RSV seasons in infants and children who have severe immunodeficiency. Nirsevimab is preferred over palivizumab. If nirsevimab is not available, palivizumab should be used according to the NACI Statement on the Recommended use of palivizumab to reduce complications of respiratory syncytial virus infection in infants (2022).
There are efficacy and safety data to support the use of the RSV monoclonal antibody nirsevimab in infants and children with immunocompromise.
Being immunocompromised is not a precaution to immunization with RSVpreF. However, there are no data on its use in pregnant women and pregnant people with immunocompromise. They may have a diminished immune response to the vaccine.
Refer to Immunization of immunocompromised persons in Part 3 for additional general information about vaccination of people who are immunocompromised.
RSV monoclonal antibodies should be provided to infants and children whose transportation for severe RSV disease treatment is complex. Nirsevimab is preferred over palivizumab. Nirsevimab should be prioritized for infants entering, or born during, their first RSV season whose transportation for severe RSV disease treatment is complex.
Nirsevimab should be prioritized for infants entering, or born during, their first RSV season whose risk of severe RSV disease intersects with established social and structural health determinants which due to social, environmental, and economic factors, rooted in the history of colonization and systemic racism (i.e., structural inequity) are experienced by some Indigenous communities across First Nations, Métis and Inuit populations. This applies to infants who live in or are part of First Nations, Métis, and Inuit communities. Nirsevimab is preferred over palivizumab.
If nirsevimab is not available, palivizumab should be used according to the NACI Statement on the Recommended use of palivizumab to reduce complications of respiratory syncytial virus infection in infants (2022) as follows: Palivizumab should be offered to infants less than 36 wGA and less than 6 months of age living in remote northern Inuit communities who would require air transport for hospitalization. Palivizumab is not routinely recommended for healthy full term infants living in remote northern Inuit communities. Palivizumab may be considered for these infants if aged less than 6 months and living in a community with a very high RSV hospitalization rate, taking into consideration the burden of illness in the community and need for air transport if hospitalization or specialized ambulatory care is required.
Palivizumab may be considered for infants less than 36 wGA and less than 6 months of age living in other remote communities with documented high rates of hospitalization for RSV who would require air transport for hospitalization.
To prevent severe RSV disease in their infant, a pregnant woman or pregnant person, in conversation with their pregnancy care provider, might consider immunization with RSVpreF vaccine in advance of, or during, the RSV season.
RSVpreF vaccine should be given at 32 through 36 weeks gestation to allow time for the development of an immune response and passive antibody transfer to the fetus before birth. RSVpreF needs to be administered at least 2 weeks before birth to allow for the transplacental transfer of protective antibodies.
There is no evidence, but it is possible, based on other vaccines studies, that there would be a modest transfer of protective antibodies through breast milk if the breastfeeding woman or breastfeeding individual received vaccine in pregnancy or during breast feeding.
To reduce morbidity and mortality associated with RSV infection in older adults, a single dose of RSV vaccine, either RSVPreF3 or RSVpreF, is recommended for adults 75 years of age and older, particularly older adults at increased risk of severe RSV disease (see List 2 ).
Adults 60 years of age and older who are residents of nursing homes and other chronic care facilities are among those at highest risk for severe outcomes from RSV disease. They should be vaccinated with either RSVPreF3 or RSVpreF vaccine.
Adults 60 to 74 years of age, and not among the recommended groups for publicly funded RSV vaccine programs, may consider RSV immunization in consultation with their healthcare provider. The duration of protection of the RSV vaccine is not yet known, and it is unclear if the protection offered by vaccination can be boosted by subsequent doses. Therefore, healthy individuals who are less than 75 years of age may want to discuss deferring vaccination with their health care providers to a future time when they may be at greater risk of severe RSV disease and the vaccine may confer greater benefit.
Adults who live in or are part of some First Nations, Métis, and Inuit communities might consider RSV vaccination at a younger age given the evidence for an increased burden of illness due to social, environmental, and economic factors, rooted in the history of colonization and systemic racism (i.e., structural inequity).
Dose and route of administration
For infants entering their first RSV season, nirsevimab is administered as a single dose of 50 mg/0.5 mL for infants weighing less than 5 kg, and a single dose of 100mg/1 mL for infants weighing 5 kg or more. For most children entering their second RSV season who are at ongoing risk of severe RSV disease, nirsevimab 200 mg (2 x 100 mg/1 mL) is given in 2 different injection sites. However, if the child weighs less than 10 kg entering their second RSV season, consideration can be given to administering a single dose of 100 mg at clinical discretion.
Palivizumab is given at a dose of 15 mg/kg of body weight, approximately every 28 days, during RSV season, for a total of 4 or 5 doses.
RSVpreF (Abrysvo TM ) vaccine is administered as a single 0.5 mL dose in pregnancy between 32 and 36 weeks gestation.
For adults 60 years of age and older, RSVPreF3 (Arexvy) or RSVpreF (Abrysvo TM ) is administered as a single 0.5 mL dose.
Route of administration
Monoclonal antibodies are administered by intramuscular (IM) injection to infants and children.
RSVpreF (Abrysvo TM ) vaccine is administered IM to pregnant women and pregnant people. RSV vaccines are administered IM in adults.
Refer to Vaccine administration practices in Part 1 for additional information about pre-vaccination and post-vaccination counselling, vaccine preparation and administration technique and infection prevention and control.
Concurrent administration with other vaccines
Nirsevimab and palivizumab are passive immunizing agents directed specifically against RSV. These monoclonal antibodies do not interfere with the immune response to other vaccines. Nirsevimab or palivizumab can be administered at the same time as, or at any time before or after, other immunization products.
RSV vaccines can be administered at the same time as, or at any time before or after, other vaccines. However, if possible, RSV vaccine should be given at least 6 weeks before or after non-seasonal vaccines, for example, shingles or diphtheria-tetanus vaccines, to avoid inadvertently attributing an adverse event from another vaccine to the RSV vaccine.
Some studies suggest that concurrent administration of the RSV and influenza vaccines may result in a lower immune response, but the clinical significance of this is unknown.
Refer to Blood products, human immunoglobulin and timing of immunization and Timing of vaccine administration in Part 1 for additional information.
RSV monoclonal antibodies should be stored between 2°C and 8°C in their original containers and must not be frozen.
RSV vaccines should be stored in a refrigerator between 2°C and 8°C in their original containers to protect them from light. RSV vaccines must not be frozen.
For additional information, consult the product monographs available through Health Canada's Drug Product Database . Refer to Storage and handling of immunizing agents in Part 1 for additional information.
Common and very common adverse events
Common adverse events occur in 1% to less than 10% of vaccinees. Very common adverse events occur in 10% or more of vaccinees. Adverse events following administration of RSV monoclonal antibodies are uncommon.
In randomized controlled trials the rates of local and systemic adverse events were similar for those receiving either nirsevimab or palivizumab as for those receiving a placebo.
The most-reported adverse events following immunization with RSVpreF vaccine in pregnant women and pregnant individuals were pain at the injection site, headache and myalgia.
Overall, RSVpreF and RSVPreF3 are well-tolerated in adults 60 years of age and older. The most common adverse events after RSV vaccination in older adults are usually mild or moderate and include pain at the injection site, fatigue, headache, and muscle or joint pain.
Uncommon, rare and very rare adverse events
Uncommon adverse events occur in 0.1% to less than 1% of vaccinees. Rare and very rare adverse events occur, respectively, in 0.01% to less than 0.1% and less than 0.01% of vaccinees. In infants, fever and/or rash at the injection site occurred at a rate of 0.5% within 7 days following administration of RSV monoclonal antibodies. Compared with placebo, nirsevimab does not increase the risk of severe systemic adverse events in infants and RSVpreF vaccine does not increase the risk of severe systemic adverse events in pregnant women and pregnant people or their infants.
Serious adverse events following administration of palivizumab, mostly hypersensitivity reactions, are rare at 1.3 to 3.4 per 10,000 doses administered. Anaphylaxis occurs in approximately 1 per 1 million doses, similar to the rate seen with immunization in general.
Other safety issues
Repeated injections of a humanized monoclonal antibody have raised concern for the development of immune mediated disease. However, studies have not shown an increased risk of autoimmune disease or atopy in children exposed to palivizumab.
Some studies have found an increase in preterm births among RSVpreF vaccine recipients compared to placebo recipients. This was not observed in high-income countries like Canada. It is unclear whether there is a causal relationship with the vaccine; the currently available data are inconclusive. By limiting vaccine administration to 32 through 36 weeks gestation, the potential risk of preterm birth is reduced. NACI will continue to monitor the RSVpreF vaccine safety data and will update its recommendation if needed.
Safety data are limited among adults 60 years of age and older. However, early safety data suggest a potential increased rate of atrial fibrillation events and inflammatory neurologic events, including Guillain-Barré syndrome, after administration of RSVpreF or RSVPreF3 vaccines in adults 60 years of age and older. Information currently available is insufficient to determine if there is an increased frequency of these events associated with the vaccines. NACI will continue to carefully monitor the evidence on the safety of RSVpreF and RSVPreF3 vaccines in adults and will update guidance accordingly.
Appropriate vaccine administration is essential to the optimal safety and efficacy of vaccines. To avoid administration errors where RSV vaccines are inadvertently administered to the wrong populations, for example, children being given the RSVpreF vaccine, refer to Vaccine administration practices chapter, Table 2: Vaccine provider administration check list .
Guidance on reporting adverse reactions following RSV monoclonal antibody administration and adverse events following immunization (AEFI)
To ensure the ongoing safety monitoring in Canada of passive immunizing agents such as palivizumab and nirsevimab, and the active immunizing agents, RSVpreF and RSVPreF3, reporting of adverse reactions and events by health care providers is critical. In some jurisdictions, reporting is mandatory under the law.
Vaccine providers are asked to report AEFIs through local public health officials and to check for specific AEFI reporting requirements in their province or territory. In general, any serious or unexpected adverse event believed to be temporally related to vaccination should be reported.
When a serious or unexpected adverse reaction follows the administration of a passive immunizing agent such as palivizumab or nirsevimab, report the adverse drug reaction to the Canada Vigilance Program using the Side Effect Reporting Form available on the program web page. The Canada Vigilance Program collects and assesses reports of suspected adverse reactions to health products, including biologics. If palivizumab or nirsevimab was given along with an active immunizing agent, the adverse event(s) should also be reported through local public health officials .
Refer to Vaccine Safety and Pharmacovigilance and Adverse Events Following Immunization in Part 2 for additional information on vaccine safety. Refer to Contents of immunizing agents authorized for use in Canada in Part 1 for additional information on the components of nirsevimab, palivizumab and RSVpreF vaccine.
Contraindications and precautions
Nirsevimab, palivizumab, and RSVpreF and RSVPreF3 are contraindicated in individuals with a known hypersensitivity or history of a severe allergic reaction (e.g., anaphylaxis) to any component of the products. A known hypersensitivity to other humanized monoclonal antibodies is also a contraindication for nirsevimab and palivizumab. Refer to Contraindications and precautions chapter for more information on allergies and to Contents of immunizing agents authorized for use in Canada for a list of all vaccines authorized for use in Canada and their contents.
Minor illnesses such as the common cold, with or without fever, are not contraindications to use of RSV monoclonal antibodies or RSV vaccines. Moderate to severe illness, with or without fever, is a reason to consider deferring administration. The decision to delay administration of an immunizing agent will depend on the severity and etiology of the underlying disease as well as the risk of not immunizing.
This chapter was revised based on the National Advisory Committee on Immunization's (NACI) Statement on the prevention of respiratory syncytial virus (RSV) disease in older adults .
This chapter has been revised based on the NACI Statement on the prevention of respiratory syncytial virus (RSV) disease in older adults prepared by N Brousseau, A Killikelly and W Siu on behalf of the NACI RSV Working Group. The chapter was prepared by F Crane, and reviewed by E Abrams, C Jensen, and N Brousseau.
The CIG gratefully acknowledges the contribution of: N Haddad and A Howarth.
Abrams EM, Doyon-Plourde P, Davis P, Brousseau N, Irwin A, Siu W, et al. Burden of disease of respiratory syncytial virus in infants, young children and pregnant women and people. Canada Communicable Disease Report. 2024 Feb; 50(1/2):1-15. https://doi.org/10.14745/ccdr.v50i12a01
American Academy of Pediatrics. Respiratory Syncytial Virus. In: Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH (Eds). Redbook: 2021 Report of the Committee on Infectious Diseases. Itasca IL. American Academy of Pediatrics: 2021. (pp 628-636).
AstraZeneca Canada Inc. Product monograph - SYNAGIS ® July 9, 2021.
Sanofi Pasteur Limited. Product monograph – Beyfortus ® April 19, 2023.
Health Canada. Canada Vigilance Program. Accessed: December 02, 2022 from https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/canada-vigilance-program.html
GlaxoSmithKline Inc. Product monograph – Arexvy August 4, 2023.
National Advisory Committee on Immunization. An Advisory Committee Statement: Statement on the prevention of respiratory syncytial virus (RSV) disease in infants. May 17, 2024. Accessed May 17, 2024 from: https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/national-advisory-committee-immunization-statement-prevention-respiratory-syncytial-virus-disease-infants.html
National Advisory Committee on Immunization. An Advisory Committee Statement: Statement on the prevention of respiratory syncytial virus (RSV) disease in older adults. August 9, 2024. Accessed August 9, 2024 from: https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/national-advisory-committee-immunization-statement-prevention-rsv-disease-older-adults.html
National Advisory Committee on Immunization. An Advisory Committee Statement: Recommended use of Palivizumab to Reduce Complications of Respiratory Syncytial Virus Infection in Infants. June 1, 2022. Accessed November 25, 2022 from: https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/vaccines-immunization/palivizumab-respiratory-syncitial-virus-infection-infants/palivizumab-resp-infection-infants-eng.pdf
Pfizer Canada Product monograph – Abrysvo TM December 21, 2023.
Public Health Agency of Canada. Respiratory Viruses Detections in Canada. Accessed March 2024 from: https://www.canada.ca/en/public-health/services/surveillance/respiratory-virus-detections-canada.html
Walsh E, Marc GP, Zareba A, et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. The New England Journal of Medicine. 2023 Apr 20;388(16):1465-1477. http://doi.org/10.1056/NEJMoa2213836
Autonomous decisions should be made by Indigenous Peoples with the support of healthcare and public health partners in accordance with the United Nations Declaration on the Rights of Indigenous Peoples Act .
Page details
Home / IAVI Report / RSV vaccines: the latest success story
March 14, 2023
RSV vaccines: the latest success story
After decades of research, structure-based design efforts are yielding effective vaccines against respiratory syncytial virus. jason mclellan explains the breakthroughs that paved the way..
Kristen Kresge Abboud
Respiratory syncytial virus (RSV) seemed an easy foe. Not long after the virus was isolated from a chimpanzee with a respiratory infection and determined to be of human origin, vaccine development efforts were underway (see timeline on RSV vaccine development below ).
Approximately 64 million people worldwide suffer from respiratory tract infections caused by RSV every year, but the virus is most serious in the very young and the elderly. Nearly 3 million children under the age of five and more than 300,000 older adults are hospitalized annually due to RSV infection, which in some cases is fatal. RSV poses an even greater risk of death to children under one year of age than influenza.
But whatever optimism there was that a vaccine would be readily developed faded quickly in the mid-1960s when a whole-killed RSV vaccine candidate led to enhanced disease among infants who were subsequently exposed to naturally circulating virus, killing two children and sending others to the hospital. Following that, the focus shifted to understanding the immunological determinants of what had gone wrong, what researchers came to refer to as RSV-vaccine-associated enhanced respiratory disease.
Then, in 2013, a critical discovery put researchers on a new track to developing an RSV vaccine, this time backed by structure-based vaccine design. This discovery was made by Jason McLellan, a structural biologist who at the time was completing his postdoctoral studies at the Vaccine Research Center (VRC) at the National Institute of Allergy and Infectious Diseases (NIAID) at the U.S. National Institutes of Health (NIH).

McLellan came to the VRC and began working on HIV but soon shifted his focus to RSV when he teamed up with veteran infectious disease researcher Barney Graham, who had already been working on RSV for decades by that time.
Working backwards from an antibody to RSV, McLellan and colleagues identified sites on the surface protein of the virus, known as F, that are particularly vulnerable to attack by neutralizing antibodies, making it an ideal vaccine immunogen.
The problem was that the F protein was very unstable, flipping easily from one conformation to another, and it was only in one specific conformation, known as prefusion, that these highly desirable parts of the protein known as epitopes were exposed. Once the F protein is in the postfusion state, these epitopes are hidden.
To engineer the F protein as a vaccine immunogen, researchers therefore needed to lock it in the prefusion conformation. In 2013, McLellan and colleagues reported that they did exactly that . It ended up being one of the scientific breakthroughs of the year in Science magazine, where editors had this to say: “Researchers have long hoped that structural biology, the study of the molecules of life, would help them design better vaccines. This year, it began to deliver.”
This discovery formed the basis for many of the RSV vaccine candidates that are now in development, two of which may soon be authorized by the U.S. Food and Drug Administration — one from Pfizer , the other from GSK . It also led to the development of the stabilized Spike protein of SARS-CoV-2 that was used in the first COVID-19 vaccines.
Where it will lead to next is anybody’s guess.
You might say McLellan, now a professor at the University of Texas at Austin , has a knack for solving structures, and it turns out that these structures are giving way to incredibly important vaccines. I spoke with McLellan about the arc of RSV vaccine development and how structural biology and machine learning together are heralding a new era of vaccines.
An edited version of our conversation appears below.
What was the key to unlocking the prefusion conformation of the RSV F protein and why was it so difficult to do?
It was difficult just because the RSV F protein is so unstable. The prefusion conformation is what is referred to as meta-stable, which means it is not its lowest energy conformation, and so there is a kinetic barrier keeping it from staying in this state. For some viral fusion proteins this barrier can be quite high, and RSV may be one of the most unstable viral fusion proteins in its class. Even just purifying the virus triggers it to go into the postfusion conformation, but we don’t actually know what the specific trigger is. For HIV, for example, HIV Envelope binding to its receptor on cells induces a conformational change, but for RSV we don’t know what the receptor is or even if there is one for the RSV F protein.
The F protein is just very unstable, and to stabilize it in its prefusion state we first needed to come up with some method of obtaining a reasonably high resolution structure that we could use to engineer in amino acid substitutions to help stabilize it in that conformation. And to do that, the trick was to identify monoclonal antibodies that could bind exclusively to the prefusion conformation and prevent the protein refolding from occurring.
The fact that the receptor for the F protein, if there is one, isn’t known makes it sound as though there are some gaps in our understanding of the virology of RSV. Is that true?
There are a lot of gaps in the virology. I think it’s kind of amazing that we’ll have a vaccine before we really figure out the molecular details of entry for the virus.
How did you identify the antibodies to RSV that allowed you to do the structural work that was required?
It started with a really nice paper in 2011 from Jose Melero and colleagues. They took serum from people that had high titers of RSV-neutralizing antibodies and passed it over a column mobilized with the postfusion form of F. Antibodies specific to the postfusion form would stick to the column, but what they found was that most of the neutralizing antibodies passed through the column without sticking, suggesting they bound to some other conformation. That paper was key.
Based on that, we set out to obtain one or more prefusion-specific binding antibodies, and we eventually found one mouse antibody and one human antibody that bound only the prefusion conformation, not the postfusion.
The next step was to trap one of these antibodies in complex with the prefusion F. To do that, I expressed the unstable F protein in the presence of the human antibody called D25. The idea was that the antibody would bind, lock it in place, and then we could purify the complex of an antibody-bound prefusion protein, and this actually worked really well.
A derivative of this antibody, Nirsevimab , will likely be approved this year as a prophylactic antibody for infants.
Then you needed to identify the epitopes on the prefusion F that were susceptible to antibodies, right?
Yes. We knew that all the antibodies to the prefusion F protein bound to the apex. This was a novel site that nobody had seen before, in part because nobody had identified the prefusion F structure, and this is the region that completely refolds into a different conformation in the postfusion form so immunizing with postfusion F is never going to elicit these antibodies. The goal then was to use structure-based design to engineer amino acid substitutions into the F protein that help lock it in the prefusion state and prevent the conformational change from occurring.
This worked too, and when you immunized animals, you induced neutralizing antibodies that could protect, which makes me think about HIV. Even after stabilizing the HIV Envelope protein it still doesn’t induce the types of neutralizing antibodies that are protective. Luckily that wasn’t the case with RSV.
We’ve learned a lot of lessons from RSV, but they don’t help overcome the issues plaguing HIV vaccine development. When I joined Peter Kwong’s lab at the VRC, the whole lab was focused on HIV vaccine design and structure-based vaccine design, and I thought we were coming up with cool ideas for protein stabilization and epitope scaffolding. But nothing worked. And when that happens it’s hard to know if the ideas are flawed, or if HIV is just such a difficult virus. So I decided to try some of these approaches with a more tractable virus and Barney suggested that RSV was a perfect test case. And it was a terrific test case to show that structure-based vaccine design can work.
Trying to create an HIV vaccine is incredibly difficult but in the process of trying to do so you are going to spin off cool technologies. Many other fields have benefitted from the advances in structure-based design, B-cell sorting, and antibody isolation that were pioneered for HIV.
Which is what happened with COVID also.
That’s right. We published two papers on the proof of concept for structure-based vaccine design with RSV in 2013 and this work was one of Science magazine’s top 10 breakthroughs of the year . We thought RSV was going to be the first vaccine developed based on this work, but, of course, it took a decade to actually develop the vaccine candidates and COVID came along in the meantime.
Fortunately, we were prepared for that because after RSV we started working on the Middle East respiratory syndrome (MERS) coronavirus. We couldn’t initially obtain the structure of the MERS Spike protein, but we did obtain a structure of the Spike protein from another human coronavirus, HKU1. Using this structure, we engineered stabilizing substitutions that worked for MERS Spike, as well as related betacoronavirus Spikes. These substitutions also increased Spike expression, and this meant we were well prepared to do the same thing when SARS-CoV-2 emerged, which is partly why vaccines were developed so rapidly.
So where do you go next?
Basically everywhere — the sky’s the limit. And it’s not just viruses. People are starting to do the same thing now for bacterial pathogens and parasites, such as malaria. It’s not going to solve all the problems, but I think it’s clearly become a very important tool for vaccine developers, along with all the new advances in machine learning. It’s a super exciting time.
How could machine learning or AlphaFold help you with your work on designing vaccine immunogens?
I think there’s a lot of enthusiasm about sort of running AlphaFold backwards, if you will. For AlphaFold the input is a sequence, and the output is a protein structure. We’re interested in the input being a protein structure and the output being a sequence that would help us identify amino acid substitutions that would stabilize the structure.
Right now, we do almost all of it by eye or by using some programs, but we might have to design 100 stabilizing substitutions and test them all individually to get 20 or 25 that work. We’d love it if machine learning could cut that down to testing 50 and getting 25 that work.
And what’s even more exciting would be if a machine-learning program can come up with substitutions we haven’t even thought of through things it has learned. There’s a lot of excitement with everything that’s going on with that. I think it’s a peak time in the next 10 years where we will get a burst of new vaccines based on these new technologies.
Persistence pays
A timeline of the key scientific progress in RSV vaccine development
1956: Respiratory syncytial virus (RSV) is isolated from a lab animal with an upper respiratory tract disease. The paramyxovirus is initially referred to as “chimpanzee coryza agent.”
1957: RSV is quickly determined to be of human origin and is found to be the cause of a common lung infection in young children and infants.
1965-1967: Following on the success of the whole, inactivated polio vaccine developed by Jonas Salk, researchers develop a whole, inactivated RSV vaccine candidate and test it in four large studies. The vaccine is not efficacious, and, in fact, causes enhanced disease in infants subsequently infected with naturally circulating RSV, temporarily derailing RSV vaccine research efforts.
1982: The viral genome of RSV is sequenced.
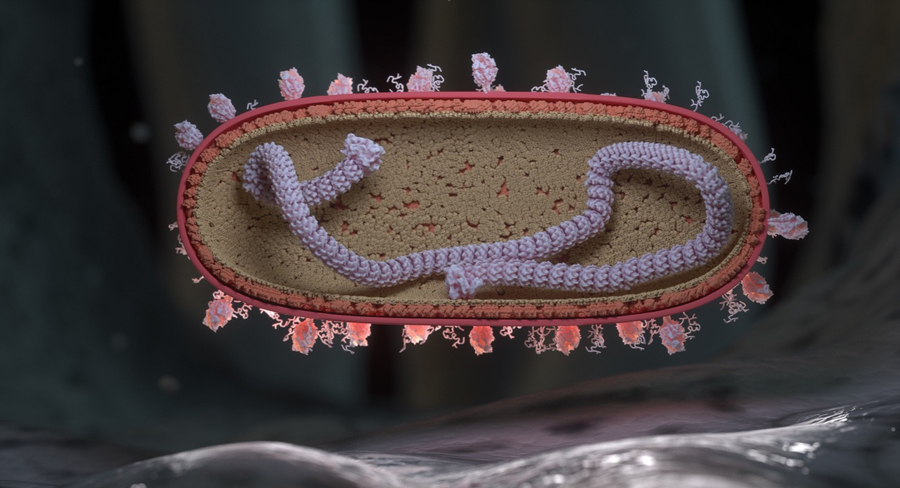
1983: Researchers isolate mouse monoclonal antibodies to RSV.
1984: The surface glycoprotein and fusion glycoprotein (F) of the virus are isolated.
1986: Recombinant RSV proteins are derived from poxvirus vectors.
1989: Recombinant RSV F protein is derived from insect cells.
1990s/early 2000s: Small animal models are used to elucidate the immunological determinants of RSV-vaccine-associated enhanced respiratory disease. At the same time, researchers expand their understanding of the role of T cells in RSV pathogenesis.
Following this, vaccine research efforts are “cautiously reinitiated.” Five Phase III trials of vaccine candidates based on the prefusion conformation of the RSV F protein are conducted but none are efficacious enough.
1997: Researchers show that passive administration of a human monoclonal antibody known as palivizumab can protect against severe disease from RSV.
2006: The first atomic-level structure is published of a paramyxovirus F protein in its prefusion conformation.
2012: Researchers show that a major target of antibodies induced by natural RSV infection is on the prefusion conformation of the virus’s F protein, making this less stable form of the viral protein an obvious target for vaccine design efforts.
2013: McLellan and colleagues at the VRC at NIAID at the NIH create a stabilized version of the RSV F protein in its prefusion conformation, kicking off vaccine development efforts based on the prefusion F protein.
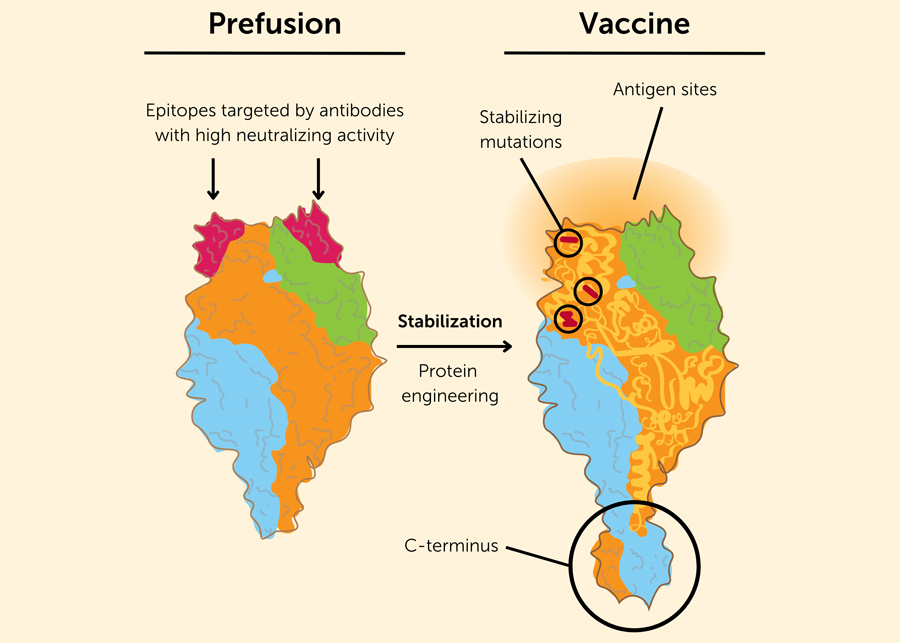
2019: Structure-based vaccine design work used to stabilize the RSV F protein in its prefusion conformation are applied to coronaviruses in response to outbreaks of Middle East respiratory syndrome (MERS).
2020: When SARS-CoV-2 emerges, researchers use similar techniques identified through work on RSV to stabilize the Spike protein of the virus, leading to the development of highly efficacious COVID-19 vaccines in record time.
2023: Advisers to the U.S. Food and Drug Administration recommend that the agency approve two RSV vaccine candidates for older adults, one developed by Pfizer and the other by GSK .
- The Journey to RSV Vaccines , Barney S. Graham, New England Journal of Medicine .
- Structure-Based Design of a Fusion Glycoprotein Vaccine for Respiratory Syncytial Virus , McLellan et. al., Science ; 2013 November 1; 342(6158): 592–598.
- Respiratory syncytial virus vaccine development , Julia L. Hurwitz, Expert Review Vaccines ; 2011 October; 10(10): 1415-1433.
Select Your Interests
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
RSV Infection in Older Adults
- 1 University of Michigan Health System, Ann Arbor
- 2 Deputy Editor, JAMA
- Medical News & Perspectives RSV Vaccines, Finally Within Reach, Could Prevent Tens of Thousands of Yearly Deaths Jennifer Abbasi JAMA
- Medical News in Brief FDA Clears RSV Vaccine for Adults Aged 60 Years or Older Emily Harris JAMA
- Medical News in Brief CDC: RSV Vaccine Recommended for Older People Emily Harris JAMA
- JAMA Insights Therapies to Decrease Severe Respiratory Syncytial Virus Illness Elizabeth A. Scruggs-Wodkowski, MD; Preeti N. Malani, MD, MSJ; Kathleen A. Linder, MD JAMA
Respiratory syncytial virus (RSV) causes respiratory infections in humans.
What Are the Symptoms of RSV Infection?
In most people, RSV causes mild cold-like symptoms of cough, runny nose, sore throat, headache, decreased appetite, and fever that typically last less than 5 days. However, in older adults, RSV can cause more severe disease such as pneumonia, or can worsen respiratory diseases, including asthma or chronic obstructive pulmonary disease (COPD).
Timing of RSV Infections and Incidence of Severe Infection
RSV infections typically begin in the fall and peak during the winter. Each year in the US, RSV causes about 60 000 to 160 000 hospitalizations, and up to 10 000 deaths in adults older than 60 years.
Risk Factors and Spread of RSV Infection
Adults at highest risk of severe RSV infection include those older than 60 years with any of the following conditions: lung disease (such as COPD and asthma), heart disease (such as heart failure and coronary artery disease), diabetes, neurological diseases, kidney disease, liver disease, blood disorders, or immunosuppression. People who live in a nursing home or long-term care facility are also at high risk of severe RSV infection.
RSV spreads from person to person through the airborne route when someone coughs or sneezes, or by direct contact through the nose, mouth, or eyes after a person touches an infected surface. People with RSV can spread the virus for up to 2 days before they feel sick and may be contagious for 3 to 8 days after they develop RSV symptoms.
Measures to Reduce Risk of RSV Infection
RSV infection can be decreased if people wash their hands, cover their coughs and sneezes, and avoid close contact such as kissing, shaking hands, and sharing drinking glasses with others. Disinfecting surfaces and encouraging individuals with cold-like symptoms to remain at home also decrease the risk of RSV transmission.
Vaccination for RSV
The US Food and Drug Administration (FDA) recently approved 2 new RSV vaccines, Arexvy and Abrysvo. Both vaccines are moderately to highly effective in preventing severe RSV infections in adults aged 60 years or older.
RSV vaccination is currently given by a single injection, although studies are ongoing to determine if additional booster doses should be recommended. RSV vaccine can be given at the same time as other vaccines, such as influenza or COVID-19. Individuals with a minor illness, such as a cold, can be vaccinated, but RSV vaccination should be delayed for those who are moderately or severely ill. RSV vaccine should not be given to people with a prior severe allergic reaction to any component of the vaccine.
The Centers for Disease Control and Prevention (CDC) recommends that adults older than 60 years discuss RSV vaccination with their clinician. People who are at high risk of developing severe RSV infection based on their age and medical conditions should strongly consider receiving an RSV vaccine.
After RSV vaccination, people may have pain, redness, and swelling in the area where the shot was given, and some individuals may experience fatigue, fever, headache, nausea, diarrhea, and muscle or joint pain. Severe allergic reactions to RSV vaccines are very rare.
For More Information
Centers for Disease Control and Prevention www.cdc.gov/vaccines/hcp/vis/vis-statements/rsv.html
To find this and other JAMA Patient Pages, go to the Patient Information collection at jamanetworkpatientpages.com .
Published Online: September 7, 2023. doi:10.1001/jama.2023.16932
Conflict of Interest Disclosures: None reported.
Source: Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep . 2023;72(29):793-801. doi:10.15585/mmwr.mm7229a4
See More About
Linder KA , Malani PN. RSV Infection in Older Adults. JAMA. 2023;330(12):1200. doi:10.1001/jama.2023.16932
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
August 9, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
COVID, flu and RSV: What to know about who should get vaccinated and when
by Laura Williamson, American Heart Association News
It has been more than a year since the World Health Organization declared an end to the COVID-19 pandemic. But while the virus no longer qualifies as a crisis, experts say it will only stay under control if people get vaccinated.
"Population immunity has moved us out of the pandemic," said Dr. Manisha Patel, chief medical officer for the Centers for Disease Control and Prevention's National Center for Immunization and Respiratory Diseases. "Now the goal is to make sure we keep that immunity up because it does wane. And the way we keep it up is through vaccination. That is the safest way to keep our country healthy."
The CDC recently announced that new COVID-19 vaccines would be available later this year. The agency recommends everyone—with the exception of babies under 6 months old—get vaccinated to protect against serious illness this fall and winter. The recommendation applies to people who have previously been vaccinated as well as to those who have never had a COVID-19 vaccine.
People who recently had COVID-19 can wait three months before getting vaccinated, the CDC says. However, certain people may consider getting the vaccine sooner, including those at risk for severe COVID-19.
"Since the 2024–2025 COVID vaccines won't be available until the early fall, it is still fine to get the 2023-2024 vaccine for people who need to be protected now," Patel said.
The CDC also updated its vaccine recommendations for the flu and respiratory syncytial virus , or RSV, as it gears up for the spread of respiratory infections that typically starts in the fall.
"These are some of the most commonly circulating viruses and they start to peak in the fall and winter season," Patel said. "We time the release of the vaccines to make sure people are getting optimum protection."
In the U.S., 81% of adults have had at least one dose of a COVID-19 vaccine, but only 21% got the updated 2023–24 vaccine, according to CDC data. Only 9% said they would definitely get the 2023-24 vaccine, and 43% said they probably or definitely would not.
That could leave many people unprotected against currently circulating strains of the virus, said Dr. Hung Fu Tseng, a research scientist at the Kaiser Permanente Department of Research and Evaluation and a professor at the Kaiser Permanente Bernard J. Tyson School of Medicine in Pasadena, California.
"Our protection goes away over time," he said, adding that a regular vaccine dose is needed to keep that protection.
Like the flu vaccine, COVID-19 vaccines will offer protection against currently circulating variants during the fall and winter, Patel said. "They should protect you through the season."
The main goal of vaccination—for any virus—is not to completely prevent infection but to reduce disease severity if an infection occurs, Tseng said. "The goal is to prevent severe outcomes, especially for the elderly, immune-compromised people and infants."
Studies show those protections peak in the early weeks following flu and COVID-19 vaccination and then slowly wane, but remain effective for five months or longer.
Vaccines can "bring the disease from wild to mild," Patel said. Whether they prevent someone from developing symptoms depends on numerous factors, including age and whether the person has any underlying conditions.
COVID vaccination clearly makes a difference in disease severity and the risk of being hospitalized, even in younger adults. CDC data shows that 78% of adults under 50 who were hospitalized because of COVID-19 from October 2023 to March 2024 had not received a bivalent booster introduced in 2022 or a 2023–24 vaccine. Overall, only 11% of adults hospitalized for COVID-19 had received a 2023–24 vaccine prior to being hospitalized.
People with cardiovascular disease , which includes heart disease and stroke, are at especially high risk. Federal data shows it is the most common underlying condition shared by adults ages 50 and older who end up in the hospital because of COVID-19. "The best way to protect them is getting their COVID-19 shot," Patel said.
Having cardiovascular disease increases the risk of disease severity for all three viruses, Patel said. People 50 and older with coronary artery disease are 2.4 times more likely to be hospitalized with RSV than people without coronary artery disease, CDC data shows. And studies have linked both RSV and the flu to an increased risk for heart attacks and strokes, especially among older adults .
The CDC recommends that adults who did not get an RSV vaccine last year get one this year if they are 75 and older or if they are 60 to 74 and at increased risk of severe disease because they either live in a nursing home or have a chronic medical condition, such as lung or heart disease.
Patel said it's still unclear how long protection from an RSV vaccine lasts and that the question is being studied. "But at this point, we do not have the data to suggest that revaccination is needed right now."
However, people should get flu and COVID-19 vaccines annually because these viruses change slightly each year and the vaccines are adjusted to better fight new variants, Patel said. As with the COVID-19 vaccine, the CDC recommends flu vaccination for anyone 6 months and older, with rare exceptions.
While the 2024–25 COVID-19 vaccine likely won't be available until September or October—and can be given at the same time as the flu vaccine—the RSV vaccine has not changed since last year and may be taken slightly earlier, toward the end of August, Patel said.
The best time to get a flu vaccine is September or October, with a few exceptions, the CDC says. Pregnant women in their third trimester are encouraged to get vaccinated in July or August to protect their unborn child from the flu during the first few months of life, when they are too young to get vaccinated.
Tseng said these vaccines are generally safe and encouraged anyone eligible to get them. "Unless you are certain you might be allergic to an ingredient in the vaccine, there's no reason you shouldn't receive them," he said.
2024 American Heart Association, Inc., distributed by Tribune Content Agency, LLC
Explore further
Feedback to editors

Study finds shingles increases risk of subsequent cognitive decline
2 hours ago

Study links life purpose to sustained functioning in U.S. veterans

Swipe up! Health apps deliver real results en masse

Researchers report potential new treatment regimens for multidrug-resistant TB meningitis

Massive biomolecular shifts occur in our 40s and 60s, researchers find
4 hours ago

Australia offers lessons for increasing American life expectancy
14 hours ago

Childhood maltreatment is associated with greater cognitive difficulties than previously thought

Lack of purpose and personal growth in older age may precede mild cognitive impairment

Two new studies show how immunotherapies collaborate to boost T cell responses in melanoma
16 hours ago

Could targeting metabolism treat blood clots in antiphospholipid syndrome?
Related stories.

CDC advice on who should get the latest COVID-19 vaccine
Dec 1, 2023
Q&A: What should you know about the new COVID-19 vaccine?
Sep 14, 2023
CDC advises updated COVID vaccine for everyone over 6 months of age
Jun 28, 2024
Complications from flu largely preventable with annual flu vaccine
Dec 4, 2023
Older Californians now eligible for another COVID-19 vaccine dose
Mar 13, 2024

Video: Expert discusses increasing levels of respiratory infections
Jan 15, 2024
Recommended for you

Algorithm achieves 98% accuracy in disease prediction via tongue color
23 hours ago

Protein in mosquito saliva shown to inhibit host immune response

Genetics of COPD important for lung function in young people, study finds

Clinical trial shows JAK inhibitor improves multiple autoimmune conditions in patients with Down syndrome
17 hours ago
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES
COMMENTS
Once infected with RSV, a person can experience one of several outcomes based on their age and underlying health, ranging from asymptomatic cases to severe sickness. Otherwise healthy younger adults typically experience symptoms similar to that of a cold, like coughing, a runny nose and a fever. According to the Centers for Disease Control and ...
RSV often feels like a common cold — and it's treated the same too. Medical experts recommend that you take these self-care steps to manage mild symptoms: Rest. Drink plenty of fluids. Take acetaminophen (Tylenol, others) as needed. Use a saline solution to rinse mucus from your nose.
An RSV vaccine is recommended for pregnant people who are 32-36 weeks pregnant with seasonal administration during September-January in most of the continental United States. In jurisdictions with seasonality that differs from most of the continental United States (e.g., Alaska, jurisdictions with tropical climates), providers should follow state, local, or territorial guidance on timing ...
Currently, CDC recommends only a single dose of RSV vaccine for all adults ages 75 and older and adults ages 60-74 who are at increased risk of severe RSV disease. Based on clinical trial data, one dose of RSV vaccine can provide protection for at least 2 years. Studies are ongoing to determine whether people ages 60 and older would benefit ...
Respiratory syncytial virus (RSV) usually causes mild, cold-like symptoms in most people, but it is the leading cause of infant hospitalization in the U.S. View All For Everyone. About How It Spreads Vaccines Immunizations to Protect Infants Symptoms RSV in Infants and Young Children ...
Health. RSV is surging. Here's what to watch for and answers about treatment options. Health experts agree that the unseasonably early surges of RSV cases, especially among children, are a ...
Pick up steam. Run a cool mist humidifier in your child's bedroom and give steamy baths. The water vapor loosens congestion. Note: Use a cool mist humidifier rather than a vaporizer, which is a burn hazard. Let honey help. If your child is at least a year old, try giving them honey to help relieve their cough.
RSV and COVID-19. Because RSV and coronavirus disease 2019 (COVID-19) are both types of respiratory viruses, some symptoms of RSV and coronavirus disease 2019 (COVID-19) can be similar. In children, COVID-19 often results in mild symptoms such as fever, runny nose and cough. For adults with COVID-19, symptoms may be more severe and may include trouble breathing.
Respiratory syncytial virus ... In this patient population RSV is responsible for ~ 1.5 million episodes of LRTI and mortality rates that range from 4% to 10% depending on the patient ... estimates that RSV vaccination will be available in the next 5-10 years. 17 This review will summarize the history and journey of RSV vaccines, ...
Eligibility for the RSV vaccination. Everyone turning 75 years old on or after the 1 September 2024 will be offered a single dose of RSV vaccine. This is because older adults are more at risk of ...
The recognition of the prevalence and burden of respiratory syncytial virus (RSV) in adults is increasing, ... The patient journey may differ depending on whether they receive care from an IDN. Importantly, IDNs and non‐IDN settings were both represented in this survey. This study has several limitations.
The journey to an RSV vaccine has been long and difficult, but now, using structure-based vaccine design, the future of RSV vaccines looks bright. In the past year there have been several successful trials published for vaccines and monoclonal antibodies to protect from severe RSV disease. A bivalent prefusion F vaccine (RSVpreF) was shown to ...
Each year, RSV infections send up to 80,000 kids under 5 to the hospital for emergency treatment. A new antibody treatment could protect the youngest kids — newborns and up infants up to 2 years ...
Abstract. After decades of work, several interventions to prevent severe respiratory syncytial virus (RSV) disease in high-risk infant and older adult populations have finally been approved. There ...
Contact: Media Relations. (404) 639-3286. [email protected]. Today, CDC updated its recommendation for the use of Respiratory Syncytial Virus (RSV) vaccines in people ages 60 and older. For this upcoming respiratory virus season, CDC recommends: Everyone ages 75 and older receive the RSV vaccine. People ages 60-74 who are at increased risk of ...
Respiratory syncytial virus was associated with significant morbidity: the median length of hospital stay was 9 days; 15% (8/53) of RSV-infected patients were admitted to the intensive care unit, and 8% (4/53) died. These findings are consistent with the literature , . Similar percentages were noted in the influenza group of our study.
The substantial toll of morbidity, mortality, and health-care expenditure associated with RSV has meant researchers have been searching for a vaccine ever since RSV was isolated in 1956. The journey to an RSV vaccine has been long and dificult, but now, using structure-based vaccine design, the future of RSV vaccines looks bright.
Spectrum of clinical illness. Most RSV infections are respiratory tract infections that present as nasal congestion, cough, low grade fever and loss of appetite and last approximately 1 to 2 weeks. Approximately 20 to 30% of infected infants develop bronchiolitis or pneumonia. Croup or otitis media may also occur.
Respiratory Syncytial Virus‐Associated Hospitalizations in Children: A 10‐Year Population‐Based Analysis in Finland, 2008-2018, Influenza and Other Respiratory Viruses, 18, 3, (2024 ...
The Journey to RSV Vaccines, Barney S. Graham, ... Respiratory syncytial virus vaccine development, Julia L. Hurwitz, Expert Review Vaccines; 2011 October; 10(10): 1415-1433. An HIV vaccine is achievable especially when developed together with the people it seeks to benefit most. To do so, it is critical that we adopt a participant-centered ...
The Centers for Disease Control and Prevention has updated respiratory syncytial virus vaccination recommendations for adults 60 and older. Adults aged 60-74 at increased risk for RSV and all aged 75 and older are recommended a single dose of the GSK, Pfizer or Moderna vaccine. Individuals who previously received a dosage should not seek another.
The JAMA Patient Page is a public service of JAMA. The information and recommendations appearing on this page are appropriate in most instances, but they are not a substitute for medical diagnosis. ... Source: Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory ...
dose of a respiratory syncytial virus (RSV) vaccine. B. Updated guidance recommending adults ages 60-74 years at increased risk of severe RSV, as described in Section 5, are recommended to receive a single lifetime dose of a RSV vaccine. Patients with a risk factor not listed in Section 5 would require a prescription from the patient's ...
INTRODUCTION. Respiratory syncytial virus (RSV) is one of the great threats to child health associated with considerable acute and long-term morbidity. 1-4 In infants and toddlers, RSV is the leading cause of viral lower respiratory tract infection (LRTI, including bronchiolitis and pneumonia) worldwide. Globally, it is estimated that RSV causes 33 new million episodes of acute LRTI in ...
The researchers performed a literature review of studies evaluating the burden of RSV-related disease in patients with COPD. They also reviewed data related to anti-RSV vaccines from ongoing monitoring of vaccine efficacy. As many as one-third of patients hospitalized with RSV have pre-existing COPD, Michaud and colleagues reported.
The CDC recommends that adults who did not get an RSV vaccine last year get one this year if they are 75 and older or if they are 60 to 74 and at increased risk of severe disease because they ...
Immunology and microbiology research has witnessed remarkable growth and innovation globally, playing a pivotal role in advancing our understanding of immune mechanisms, disease pathogenesis, and therapeutic interventions. This manuscript presents a comprehensive exploration of the key areas in immunology research, spanning from the utilisation of bacterial proteins as antibody reagents to the ...